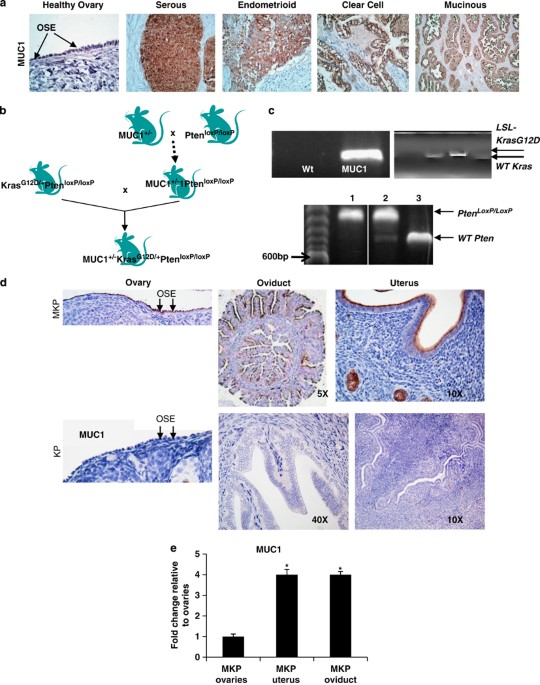
Immunobiology of human mucin 1 in a preclinical ovarian tumor model
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Epithelial ovarian cancer is an aggressive malignancy, with a low 5-year median survival. Continued improvement on the development of more effective therapies depends in part on the
availability of adequate preclinical models for in vivo testing of treatment efficacy. Mucin 1 (MUC1) glycoprotein is a tumor-associated antigen overexpressed in ovarian cancer cells, making
it a potential target for immune therapy. To create a preclinical mouse model for MUC1-positive ovarian tumors, we generated triple transgenic (Tg) mice that heterozygously express human
MUC1+/− as a transgene, and carry the conditional K-rasG12D oncoallele (loxP-Stop-loxP-K-rasG12D/+) and the floxed Pten gene (Pten/loxP/loxP). Injection of Cre recombinase-encoding
adenovirus (AdCre) in the ovarian bursa of triple (MUC1KrasPten) Tg mice triggers ovarian tumors that, in analogy to human ovarian cancer, express strongly elevated MUC1 levels. The tumors
metastasize loco-regionally and are accompanied by high serum MUC1, closely mimicking the human disease. Compared with the KrasPten mice with tumors, the MUC1KrasPten mice show increased
loco-regional metastasis and augmented accumulation of CD4+Foxp3+ immune-suppressive regulatory T cells. Vaccination of MUC1KrasPten mice with type 1 polarized dendritic cells (DC1) loaded
with a MUC1 peptide (DC1–MUC1) can circumvent tumor-mediated immune suppression in the host, activate multiple immune effector genes and effectively prolong survival. Our studies report the
first human MUC1-expressing, orthotopic ovarian tumor model, reveal novel MUC1 functions in ovarian cancer biology and demonstrate its suitability as a target for immune-based therapies.
We would like to thank Dr Daniela Dinulescu for discussion on various aspects of the mouse model, Dr Olivera Finn for critical review of the paper and Julia Thaller for technical assistance
with histology work. This study was supported by the Department of Defense Ovarian Cancer Academy Award, Pennsylvania Department of Health, Scaife Foundation and NIH/NCI 1 R01 CA163462-01
(to AMV).
Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
Department of Pathology, Magee-Women’s Hospital, University of Pittsburgh Medical Center, Pittsburgh, PA, USA
University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA
Department of Surgery, University of Pittsburgh School of Medicine, Hillman Cancer Center, Pittsburgh, PA, USA
Department of Immunology, University of Pittsburgh, Pittsburgh, PA, USA
Supplementary Information accompanies the paper on the Oncogene website
Anyone you share the following link with will be able to read this content:
