Silencing effect of CpG island hypermethylation and histone modifications on O6-methylguanine-DNA methyltransferase (MGMT) gene expression in human cancer
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

O6-methylguanine-DNA methyltransferase (MGMT) repairs the cytotoxic and mutagenic O6-alkylguanine produced by alkylating agents such as chemotherapeutic agents and mutagens. Recent studies
have shown that in a subset of tumors, MGMT expression is inversely linked to hypermethylation of the CpG island in the promoter region; however, how the epigenetic silencing mechanism
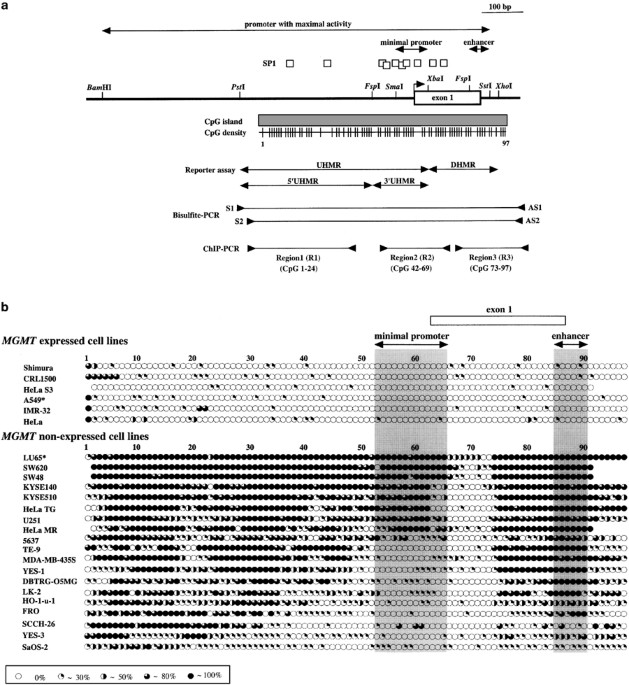
works, as it relates to hypermethylation, was still unclear. To understand the mechanism, we examined the detailed methylation status of the whole island with bisulfite-sequencing in 19 MGMT
non-expressed cancer cell lines. We found two highly methylated regions in the island. One was upstream of exon 1, including minimal promoter, and the other was downstream, including
enhancer. Reporter gene assay showed that methylation of both the upstream and downstream regions suppressed luciferase activity drastically. Chromatin immunoprecipitation assay revealed
that histone H3 lysine 9 was hypermethylated throughout the island in the MGMT negative line, whereas acetylation on H3 and H4 and methylation on H3 lysine 4 were at significantly high
levels outside the minimal promoter in the MGMT-expressed line. Furthermore, MeCP2 preferentially bound to the CpG-methylated island in the MGMT negative line. Given these results, we
propose a model for gene silencing of MGMT that is dependent on the epigenetic state in cancer.
We thank Drs M Tachibana and Y Shinkai (Kyoto University, Kyoto) for pGEX4T-G9a-C, K Sugimoto (Osaka Prefecture University, Osaka) for pGEX-H3 (1–57), and M Berne (Tufts University, Boston)
for peptide synthesis. We also thank Drs T Abe and M Oka (Yamaguchi University School of Medicine, Ube), Dr Y Shimada (Kyoto University, Kyoto) and Cell Recourse Center for Biomedical
Research Institute of Development, Aging & Cancer (Tohoku University, Sendai) for providing cancer cell lines. This work was supported by grants-in-aid for Scientific Research from the Japan
Society for the Promotion of Science (JSPS), for COE research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan, and the Uehara Memorial Foundation.
Division of Molecular Biology and Genetics, Department of Biomolecular Sciences, Saga Medical School, 5-1-1 Nabeshima, Saga, 849-8501, Japan
Tetsuji Nakagawachi, Hidenobu Soejima, Wei Zhao, Ken Higashimoto, Yuji Satoh, Shiroh Matsukura & Tsunehiro Mukai
Department of Biochemistry II, Graduate School of Medicine, Nagoya University, Japan
Division of Histology, Department of Pathology, International Medical Center of Japan Research Institute, Japan
Membrane Dynamics Project, Synchrotron Radiation Research Network, Harima Institute at Spring-8, Riken, Japan
Department of Molecular Biology, Institute of Gerontology, Nippon Medical School, Japan
Division of Neurofunctional Genomics, Medical Institute of Bioregulation, Kyushu University and Core Research for Evolutional Science and Technology, Japan Science and Technology
Corporation,
Anyone you share the following link with will be able to read this content:
