Cocaine or selective block of dopamine transporters influences multisecond oscillations in firing rate in the globus pallidus
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Previous studies have shown that direct-acting dopamine agonists modulate the multisecond oscillations which are present in globus pallidus spike trains in vivo in awake rats. To
investigate possible modulation by endogenous dopamine and by other monoamines, and by drugs with abuse potential, cocaine or selective monoamine uptake blockers were injected systemically
during extracellular recording of single globus pallidus neurons and the results analyzed with spectral and wavelet methods. Both cocaine and the selective dopamine uptake blocker GBR-12909
significantly shortened the period of multisecond oscillations, as well as increasing overall firing rate. Cocaine effects were blocked by dopamine antagonist pretreatment, as well as by
N-methyl-_D_-aspartate receptor antagonist (MK-801) pretreatment. Desipramine and fluoxetine (blockers of norepinephrine and serotonin uptake, respectively) had no significant effects on
multisecond oscillations. The results suggest that dopamine has a primary role among monoamines in modulating multisecond oscillations in globus pallidus activity, and that tonic
dopaminergic and glutamatergic transmission is necessary for normal slow oscillatory function. SIMILAR CONTENT BEING VIEWED BY OTHERS MODULATION OF DOPAMINE TONE INDUCES FREQUENCY SHIFTS IN
CORTICO-BASAL GANGLIA BETA OSCILLATIONS Article Open access 02 December 2021 INTRINSIC TIMESCALES ACROSS THE BASAL GANGLIA Article Open access 01 November 2021 IN VIVO PATCH-CLAMP RECORDINGS
REVEAL DISTINCT SUBTHRESHOLD SIGNATURES AND THRESHOLD DYNAMICS OF MIDBRAIN DOPAMINE NEURONS Article Open access 08 December 2020 MAIN Periodicities in brain electrical activity occur in
many nuclei and in many frequency ranges, and have been described in the electroencephalogram, in field potentials, and in the spiking activity of single neurons. Much of the attention that
brain periodicities have received has been directed to the range of frequencies from 1 Hz (the slow end of the delta rhythm) up to roughly 60 Hz (the fast end of the gamma rhythm). However,
recent studies have demonstrated that much slower periodic oscillations are commonly present in the spike trains of tonically firing neurons in the basal ganglia. The firing rates of a
majority of neurons in the globus pallidus (GP), the subthalamic nucleus, the substantia nigra pars reticulata and the entopeduncular nucleus oscillate _in vivo_ with slow periods, most
often in the range of 10–60 s (0.1–0.017 Hz) (Ruskin et al. 1999a; Ruskin et al. 1999c; Wichmann et al. 1999; Allers et al. 2000). These multisecond periodicities in tonic activity are
present in awake rats, but are markedly attenuated by general anesthesia (Ruskin et al. 1999a). One of the most striking characteristics of these slow oscillations is their strong modulation
by dopamine (DA) receptor activation (Ruskin et al. 1999a; Ruskin et al. 1999c; Allers et al. 2000). Systemically injected DA agonists shift oscillatory periods from mean periods of 25–30 s
to 5–15 s, depending on drug and dose. Spectral analysis reveals that DA receptor activation also increases the spectral power (i.e. the regularity) of multisecond periodicities. Therefore,
in addition to the well-characterized ability of systemically-administered DA agonists to change the mean firing rate of basal ganglia neurons, they also change basal ganglia firing
patterns at multisecond time scales. The use of selective DA receptor agonists and antagonists has shown that this modulation of slow firing pattern involves both the D1 and D2 subfamilies
of DA receptors (Ruskin et al. 1999a; Ruskin et al. 1999c). These initial studies of DAergic influences on multisecond periodicities utilized direct-acting DAergic drugs. More recently, we
have found that D-amphetamine and methylphenidate, drugs that act on monoamine transmission indirectly, also modulate slow oscillations (Ruskin et al. 2001). These drugs are noted for their
effects on attention and locomotor activity, including beneficial effects on these parameters in patients with attention deficit/hyperactivity disorder. D-amphetamine and methylphenidate
also have serious abuse potential. To further explore the potential for abused/addictive substances to affect multisecond oscillatory activity, the present study examined the effects of
cocaine on spiking activity in the GP. Since these three drugs raise levels of more than one brain monoamine, this study also tested the effects of the selective uptake blockers GBR-12909,
desipramine and fluoxetine (selective for the DA, norepinephrine (NE) and serotonin (5-HT) uptake systems, respectively) to determine the relative importance of each monoamine. In addition,
because many of the effects of direct-acting dopamine agonists on mean firing rate in the basal ganglia depend on _N_-methyl-D-aspartate (NMDA) receptor activity (Kelland and Walters 1992;
Allers et al. 1996; Huang et al. 1998), the NMDA receptor antagonist MK-801 was utilized to test for a similar dependence of the effects of monoamine uptake blockade on multisecond
periodicities. METHODS Extracellular single unit activity was recorded in the GP of male Sprague-Dawley rats (250-400 g) in accordance with the National Institutes of Health (USA) Guide for
the Care and Use of Laboratory Animals. In previous studies of GP unit activity, we have found that the incidence of multisecond oscillations was greatly attenuated when animals were under
general anesthesia (Ruskin et al. 1999a). Therefore, in the present study GP neurons were recorded in awake, immobilized rats as previously described (Ruskin et al. 1999a). Briefly, all
surgical procedures (tracheotomy, scalp incision, skull drilling) were performed with rats under halothane anesthesia. Halothane anesthesia was induced in an induction chamber, and was
maintained during surgery by administration of additional halothane as needed. To prevent discomfort, all surgical sites and pressure points were injected with the long-acting local
anesthetic mepivacaine, and ear bar tips and tracheal cannulas were coated with 2% lidocaine anesthetic gel. Corneal drying was prevented with LacriLube. A tracheotomy was performed, and the
trachea was cannulated. Rats were placed in a stereotaxic frame, and a hole was drilled in the skull over the GP. After surgical procedures were finished, halothane anesthesia was
discontinued, the animal was paralyzed with gallamine triethiodide (16 mg/kg i.v.) injected through the tail vein, and artificial respiration immediately begun with a rodent ventilator
(model 683, Harvard Apparatus). Artificial respiration rates were adjusted to maintain exhaled CO2 between 3.6 and 4.5%. Supplements of gallamine were given every 10–15 min. Body temperature
was maintained at 36-38°C with a heating pad. Glass microelectrodes (2.5-6 MΩ at 135 Hz) filled with 2 M NaCl containing 1% Pontamine Sky Blue were lowered stereotaxically through skull
holes to the following coordinates: 0.8-1.2 mm posterior to Bregma, 2.6-3.0 mm lateral to the midline, 5.0-7.0 mm ventral to dura. Fine vertical control of microelectrode placement was
accomplished with micromanipulators (MO-8, Narashige). Single unit activity was amplified (model 725, WPI) and monitored with an audio monitor and digital oscilloscope. Signals were
band-pass filtered between 250 and 5000 Hz, and spikes were identified with a voltage/window discriminator and recorded by Spike2 software (version 3.14, Cambridge Electronic Design). All
recorded GP units had biphasic +/− waveforms (type II; Kelland et al. 1995), and had baseline firing rates between 13 and 92 Hz (mean = 36 Hz). Five to six minutes of baseline activity was
recorded before the i.v. injection of experimental drugs through the tail vein. Drugs were injected as a single bolus, except for GBR-12909, which in some instances was injected in four
divided doses at 1-min intervals. Units were further recorded for 10–20 min. In some cases, a second drug was then injected and units were held for another 10 min. One unit was recorded per
rat. After data collection, Pontamine Sky Blue was iontophoresed to mark the recording site for later histological confirmation. Experimental drugs used include (−)-cocaine HCl, desipramine
HCl (Norpramin), S(−)-eticlopride HCl, fluoxetine HCl (Prozac), GBR-12909 2.HCl (Vanoxerine), haloperidol, (+)-MK-801 hydrogen maleate (Dizocilpine), and R(+)-SCH-23390 HCl. All experimental
drugs were from Research Biochemicals Inc., except for haloperidol (McNeil Pharmaceuticals). Drugs were dissolved in 0.9% NaCl, except for fluoxetine and GBR-12909 which were dissolved in
distilled water, and SCH-23390 which was dissolved as a 10X stock solution in 0.001 N HCl before dilution to final volume with saline. Spectral analysis of GP spike trains was performed by
two methods. For assessing the significance of periodic activity, 180 s data segments from pre- and post-drug epochs were analyzed with the Lomb periodogram (Kaneoke and Vitek 1996),
performed on binned spike trains. This method provides a measure of statistical significance for spectral features: spectra were tested against the null hypothesis that binned spiking
activity had a random Gaussian distribution, resulting in an exponential distribution of spectral power. This null hypothesis was rejected at the _p_ = .01 level. For spectral analysis,
spiking activity was binned with 200 ms bins (example spike trains in figures are shown binned at 500 ms), and periodicities were analyzed in the range of 1.5–60 s (0.67–0.017 Hz).
Periodicities slightly faster than 1.5 s in this preparation often relate to ventilation (Ruskin et al. 1999a). For example, of the presently recorded GP neurons, 11.4% had significant
oscillations in this range, all having periods between 0.77 and 0.84 s. This coincides with the range of ventilator speeds, and when ventilator action was recorded simultaneously during the
recording of a neuron with this type of activity (n = 5), the neuronal oscillatory period and the ventilator period were equal to within 1%. These ventilator-related oscillations were not
further analyzed. 180 s data segments were chosen from baseline, and from the 5–10 min and (when available) 15–20 min time ranges after drug injection. To assess drug effects on oscillatory
period, the period of the “main” spectral peak, i.e. the tallest (most powerful) spectral peak within the studied range, was taken from the power spectrum of each analyzed segment, and these
numbers were analyzed with ANOVA followed by _post hoc_ Dunnett's comparisons to baseline. Data segments that had no significant periodicity in the 1.5–60 s range were not entered into
this analysis. To assess drug effects on oscillatory regularity (i.e. spectral power) apart from effects on oscillatory period, the total area under the spectral curve within the 1.5–60 s
range was measured, including those cases where there were no significant spectral peaks, and these data were analyzed with repeated-measures ANOVA followed by _post hoc_ Dunnett's
comparisons to baseline. The α level for ANOVA and _post hoc_ tests was _p_ = .05. In a second method of spectral analysis, focusing on the evolution of periodic activity over time, entire
recordings or recording segments were represented as time-frequency plots of spectral power. These scalograms were based on the continuous wavelet transform and are analogous to Fourier
transform-based spectrograms. A wavelet-based approach was used to optimize time resolution across all examined frequencies, compared to the single time resolution of windowed Fourier
transform analysis. Scalograms were produced with the Time-Frequency Toolbox (http://www-syntim.inria.fr/fractales/Software/TFTB) with Matlab 5.3.1 (The MathWorks), and were computed with
the Morlet wavelet (15 s half-width at coarsest scale), with 256 frequencies being sampled within the analyzed range. Spiking activity was binned at 0.75 s, to reach a Nyquist limit of 1.5 s
(0.67 Hz). Spectral power was plotted on a log10 scale, with greater power represented by redder color. The color axis was scaled so that the power levels within the scalogram data for a
single neuron spanned the full color range (blue to red). RESULTS MULTISECOND PERIODICITIES IN BASELINE ACTIVITY As reported previously (Ruskin et al. 1999a; Ruskin et al. 1999c), the
baseline firing rate of most GP neurons recorded in awake rats oscillated at time scales of seconds to minutes (Figures 1 and 2)). These periodic variations could be large in amplitude: in
the baseline segment shown in Figure 2, Panel A, the firing rate varies up to 180% and down to 60% of the overall mean. Using the Lomb algorithm to assess the periodicity of these
oscillations (examples in Figure 2), 80% of neurons displayed oscillations in baseline firing rate (with periods between 1.5 and 60 s) that were sufficiently powerful (i.e., regular) to be
considered statistically significant. Oscillatory periods of the most powerful spectral peak from each baseline power spectrum are plotted in Figure 3. These baseline oscillatory periods
varied widely from neuron to neuron (within the 1.5–60 s range), but mean values for all treatment groups were approximately 30 s (28.2 ± 2.1 s for all neurons combined). EFFECTS OF
SELECTIVE MONOAMINE UPTAKE BLOCKERS Most recorded units had significant slow oscillations both before and after the injection of selective monoamine uptake blockers (Table 1). However, among
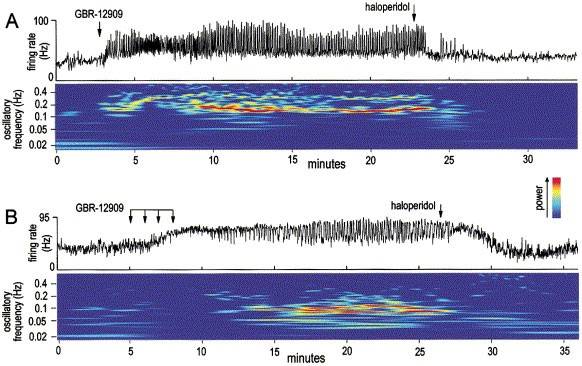
GBR-12909, desipramine and fluoxetine, only the selective DA uptake blocker GBR-12909 robustly effected these oscillations. Particularly striking oscillations appeared after the i.v.
injection of this drug (3.2 mg/kg), and, once they had appeared, could be remarkably stable (Figure 1). Spectral analyses indicated that these oscillations occurred at a faster rate compared
to baseline (Figures 1 and 3, Panel A). The mean oscillatory period within the 5–10 min post-drug epoch was ∼10 s, and this reduction in period was still significant out to 20 min after
drug injection, at which time a DA antagonist was typically administered (Figures 1 and 3, Panel A). In addition, GBR-12909 increased the power (regularity) of the multisecond oscillations.
This was visible in scalograms as the emergence of higher power bands (Figure 1), and was quantified by the measurement of spectral area in Lomb algorithm-generated power spectra. GBR-12909
increased mean spectral power by almost 70% (Figure 4). In contrast to GBR-12909, neither the NE uptake blocker desipramine nor the 5-HT uptake blocker fluoxetine had significant effects on
oscillatory period (Figure 3, Panels B and C). Also, neither drug significantly affected spectral power (data not shown). After desipramine injection, there did appear to be a modest
preponderance of oscillation periods below ∼20 s. To address the possibility that this pattern might indicate the existence of a “desipramine-responsive” subpopulation, more detailed
analyses were performed. Neurons were characterized as having high or low baseline firing rate (defined by a median split), or as responding to desipramine with increases or decreases in
firing rate (compared to baseline). Separate ANOVAs on desipramine effects on oscillatory period for each of these groups were all non-significant. In addition, post-desipramine oscillatory
period did not significantly correlate with baseline firing rate or with post-desipramine firing rate (as absolute rate or as percent of baseline). However, these data do not rule out the
possibility of a “desipramine-responsive” subpopulation of neurons or rats. In addition to effects on multisecond oscillations, GBR-12909 was the only selective monoamine uptake blocker to
have robust effects on overall firing rate. This drug increased overall firing rates in all tested neurons, whereas desipramine and fluoxetine had only minor effects (Figure 5). GBR-12909
(3.2 mg/kg) given i.v. to freely-moving rats induced a pattern of strong downward sniffing and/or chewing, sometimes combined with slow or intermittent locomotion. This pattern was present
in both the 5-10 and 15-20 min post-drug time ranges. EFFECTS OF COCAINE Striking multisecond oscillations appeared after injection of the non-specific monoamine uptake blocker cocaine
(Figure 2), and like GBR-12909, cocaine reduced the periods of these oscillations to a mean of ∼10 s within the 5–10 min epoch (Figure 2 and 3, Panel D). However, cocaine effects on
oscillatory period were short-lived, not lasting through the 15–20 min post-drug epoch, as illustrated by visual inspection of firing activity and scalograms (Figure 2), and confirmed by
Lomb analysis (Figure 3, Panel D). Cocaine-induced increases in overall firing rate were also typically short-lived, reaching a plateau at 4–9 min and dropping thereafter. These results are
consistent with the relatively short biological half-life of this drug in rats after i.v. administration (Barbieri et al. 1992). Unlike GBR-12909, cocaine did not have significant effects on
spectral power. Although some units responded to cocaine with an increase in power (e.g. Figure 2, Panel B: there is an 83% increase in spectral area (middle column) compared to baseline),
there was no consistent power response (Figure 4). Although the large majority of type II (+/− waveform) GP neurons respond to indirect-acting or direct-acting DA agonists with net increases
in firing rate, rare type II neurons are found that respond with decreased net firing rate (e.g. Figure 4 in Ruskin et al. 1998). Of the eleven GP neurons recorded during cocaine treatment,
one fell into this latter category. Notably, although cocaine caused a 66% decrease in overall firing rate in this one case, the unit still displayed the cocaine-induced reduction in
oscillatory period similar to the typical “firing rate-increasing” population (Figure 6). Similar period reductions have been noted in rare type II units demonstrating firing rate decreases
after D-amphetamine or direct-acting D1/D2 agonists (unpublished results). Because cocaine caused reductions in oscillatory period similar to those caused by the selective DA uptake blocker
GBR-12909, we attempted to confirm the DAergic mediation of cocaine effects with DA receptor antagonists. A pretreatment with combined D1 and D2 antagonists (for complete antagonism of both
DA receptor subfamilies) completely prevented the effects of cocaine on oscillatory period (Figure 3, Panel E). The combined DA antagonist treatment did not change oscillatory period by
itself (Figure 3, Panel E), but did significantly reduce oscillatory power compared to baseline. After subsequent cocaine injection, oscillatory power was no longer significantly different
from baseline (Figure 4), although it was also not significantly different from that after DA antagonist alone (_p_ = .11, Tukey test). Pretreatment with the non-competitive NMDA receptor
antagonist MK-801 also prevented the effects of cocaine on oscillatory period, without having any effect by itself on period (Figure 3, Panel F). In addition, MK-801 by itself significantly
decreased oscillatory power, and power remained reduced after subsequent injection of cocaine (Figure 4). In the 5–10 min post-drug time range, freely-moving rats injected with 3.2 mg/kg
cocaine i.v. demonstrated strong sniffing (directed downward, upward, or at the walls of the chamber), concomitant with almost continuous locomotion or rearing. Drug effects noticeably waned
by the 15–20 min post-injection time range: rats occasionally sniffed, locomoted, or groomed, but were still most of the time. DISCUSSION Previous studies from our laboratory demonstrated
that DA strongly modulates multisecond periodicities that are present in the spike trains of many GP neurons in awake rats. To extend these findings, the effects of other monoamines on GP
firing rate oscillations were investigated. The present data show that augmenting NEergic or 5-HTergic transmission alone has minor effects on multisecond periodicities, and confirm the
dominance of the DA system. These data also confirm that DA has a primary role among the monoamines for inducing increases in average firing rate in the GP (Bergstrom and Walters 1981).
Because the DA agonists used in this study are indirect-acting, i.e. they augment levels of endogenous transmitter rather than directly activate receptors, these data demonstrate that an
increase in endogenous DA is as effective in modulating the period and regularity (power) of slow oscillations in the GP as is direct activation of DA receptors by exogenous agonists. Work
with D-amphetamine and methylphenidate supports this conclusion (Baek et al. 1999; Ruskin et al. 2001). These results are therefore relevant to drug abuse and addictive processes in humans,
since abused catecholaminergic stimulants (including cocaine) act _via_ endogenous transmitter. The effects of GBR-12909 or cocaine treatment on GP firing rate and pattern, prevented and
reversed by DA receptor antagonists, are consistent with the marked increases in DA overflow caused by these drugs at or around the presently tested doses (Church et al. 1987; Baumann et al.
1994; Fink-Jensen et al. 1994; Engberg et al. 1997). However, our observations indicate that other transmitter systems are involved in these electrophysiological effects. For instance,
pretreatment with the NMDA receptor antagonist MK-801 blocked cocaine's reduction of oscillatory period, suggesting that ongoing activity at NMDA receptors is necessary for this
DA-induced effect. This type of NMDA receptor dependence has been noted for other electrophysiological effects of DA agonists in the basal ganglia (Kelland and Walters 1992; Allers et al.
1996; Huang et al. 1998), as well as for other measures of basal ganglia activity (reviewed in Huang et al. 1998). In addition, a comparison of the effects of GBR-12909 and cocaine reveals
that although both drugs reduced oscillatory period (from ∼30 s to ∼10 s), only GBR-12909 increased oscillation power. Direct-acting DA receptor agonists such as apomorphine also increase
power (Ruskin et al. 1999a). Because cocaine blocks uptake of NE and 5-HT, in addition to DA uptake, these results suggest that increased NE or 5-HT levels might diminish DA-mediated
increases in oscillatory power (while not affecting DA modulation of oscillatory period). A number of studies have demonstrated a reduction in DA-stimulated behaviors by 5-HTergic or NEergic
systems (Ungerstedt 1971; Mabry and Campbell 1973; Grabowska 1976; Fox and Brotchie 1996, 2000; Henry et al. 1998; Rocha et al. 1998; Gainetdinov et al. 1999). More generally, the present
results demonstrate that the period and power of multisecond oscillations in the basal ganglia can be independently affected, in agreement with a previous study of DA agonist effects in
another basal ganglia region, the entopeduncular nucleus (Ruskin et al. 1999b). In addition to being modulated by increased DAergic transmission, ongoing multisecond oscillations appear to
partially depend on tonic DAergic and glutamatergic activity. This dependence is reflected in the significant ∼15% decrease in oscillatory power after treatment with combined D1/D2 receptor
antagonists or a NMDA receptor antagonist. Interestingly, although these treatments blocked the effects of cocaine on oscillatory period, they did not affect oscillatory period when given
alone (similarly, oscillatory period in the subthalamic nucleus is not affected by decreased DAergic transmission due to lesion of midbrain DA neurons (Allers et al. 2000)). Since spectral
power reflects oscillatory regularity, the results suggest that tonic DAergic and glutamatergic (NMDA) tone partially supports the regularity of ongoing slow oscillations. The level of
DAergic tone in these immobilized rats is not likely to be higher than normal, since firing rates of substantia nigra pars compacta DA neurons are not significantly increased in immobilized
rats compared to anesthetized rats (Melis et al. 1998), and since, regarding multisecond oscillations, the effects of DA antagonists alone (no change in period, small-magnitude power
decrease) are minor compared with the effects of DA agonists. On the other hand, DA antagonists have striking effects when administered during pharmacologically-stimulated DA release (Figure
1). Other transmitter systems are also involved in supporting slow oscillatory power: general anesthetics (specifically, urethane and ketamine/xylazine), which affect a number of
transmitter systems, reduce spectral power in GP spike trains in the 1.5–60 s range by 50 to 60% (unpublished results), leading to a severe decrease in the incidence of statistically
significant multisecond oscillations (Ruskin et al. 1999a; Ruskin et al. 2001). The decrease in spectral power after DA antagonist administration was partially reversed by subsequent cocaine
injection. Such data suggest that the non-dopaminergic effects of cocaine can increase multisecond spectral power in the absence of DAergic tone. Together with the comparison of the effects
of GBR-12909 and cocaine (which indicate a _decrease_ of DA-stimulated multisecond spectral power), the results suggest a ‘normalizing’ effect of 5-HT or NE release on the DAergic
modulation of slow oscillatory power. This effect of 5-HT or NE appears to depend on tonic NMDA receptor activity, since there is no increase in spectral power after cocaine injection after
prior injection of MK-801. Multisecond periodicities have been noted in blood pressure (termed Mayer waves) and heart rate (Barnes and Burnham 1969; Mautner-Huppert et al. 1989). It is
possible that these peripheral oscillations might somehow drive central multisecond oscillations. However, it has recently been reported that desipramine, administered i.v. to awake rats at
one of the presently tested doses (2.0 mg/kg), induces particularly strong arterial pressure oscillations in the 10-s range (Bertram et al. 1999). Since, in the present study, desipramine
produced (at best) inconsistent effects on oscillatory period and power, it seems probable that the multisecond oscillations in activity in the basal ganglia are not simply secondary to
peripheral cardiovascular oscillations. In addition, the DA receptors that modulate basal ganglia multisecond oscillations are located centrally, not peripherally (Ruskin et al. 1999a;
Ruskin et al. 1999c). In terms of mechanisms of the DAergic modulation of slow oscillations, it is important to note that drug effects on oscillatory period were not related to drug effects
on overall firing rate. In other neuronal systems and frequency ranges, the rate of oscillatory spiking activity or the rate of periodic bursting is positively correlated with the level of
depolarization (Leresche et al. 1991; Beurrier et al. 1999; Koshiya and Smith 1999), implying a voltage-dependent mechanism. Such a relationship is not apparent for multisecond periodicities
in the GP. Although treatments that increased oscillatory frequency also increased mean firing rate in a majority of neurons, some neurons demonstrated an increased oscillatory frequency
simultaneously with a decreased mean firing rate. Additionally, in an earlier study of multisecond oscillations in the GP (Ruskin et al. 1999c), D1 agonists were found to increase
oscillatory frequency without having consistent effects on mean firing rate, while D2 agonists modestly increased firing rate without having consistent effects on oscillations. Therefore,
oscillatory period does not appear to be voltage-related for GP neurons when considered as a single population. Similar conclusions have been made about neurons in the entopeduncular nucleus
and substantia nigra pars reticulata (Ruskin et al. 1999a). Oscillatory frequency might be positively linked to depolarization in the predominant subpopulation of GP neurons that increase
average firing rate after combined D1/D2 receptor activation, and these neurons might drive (via local axon collaterals) other GP neurons to oscillate at a similar speed. However, this
scenario seems unlikely, since there is no correlation between oscillation frequency and net firing rate (either absolute or percent of baseline) after cocaine or GBR-12909 treatment in the
subpopulation with increases in average firing rate (data not shown). If oscillatory period in the GP is not related to membrane voltage, then the mechanisms by which DA modulates net firing
rate and oscillatory period in the GP must differ. Regarding the mechanism of _generation_ of slow oscillations, if these oscillations (regardless of period) can occur in the GP within a
wide range of membrane voltages, then they are likely not generated by voltage-dependent mechanisms within the GP. Instead, they might be generated in afferent structures and transmitted
synaptically to the GP, or they might arise from network mechanisms within the GP or between the GP and other structures. There is some evidence for this last hypothesis, as reverberating
oscillations have been found in organotypic co-cultures of GP and subthalamic nucleus, albeit at shorter periods (Plenz and Kitai 1999). Also, network mechanisms are clearly involved in
correlating (if not also generating) multisecond oscillatory activity, as spatially distant basal ganglia neurons can have similar periods with constant phase relationships (Allers et al.
1999; Ruskin et al. 2000). Although wavelet analysis has been increasingly utilized for analysis of electroencephalographic signals, fewer studies have used this technique for analysis of
single unit data. Many of these latter studies have utilized wavelets for spike sorting (Zouridakis and Tam 1997) or denoising (Koshiya and Smith 1999) applications, rather than for
analyzing neuronal spiking in the time-frequency domain (Przybyszewski 1991; Lewalle et al. 1995). Here, wavelet analysis successfully revealed multisecond periodicities in GP spiking
activity, and complemented our standard spectral analysis technique, the Lomb periodogram. Apart from the obvious differences in the methodologies (producing time information in the wavelet
scalogram, and statistical information in the Lomb periodogram), these two techniques routinely indicated similar oscillatory activity for a given data segment. For instance, in Figure 2,
Panel A, center, both techniques indicate a strong oscillation of ∼12 s after cocaine injection, while in the baseline epoch depicted in Figure 2, Panel B, left, both methods indicate a lack
of substantial multisecond oscillatory activity. Yet there are subtle differences between the two methods, such as the more sensitive detection of the 25-s periodicity by the Lomb
periodogram in Figure 2, Panel A, left. Possibly the most enlightening use of the wavelet scalogram for the present data is to reveal the nature of the multiple, statistically significant
spectral peaks which are present in the Lomb periodogram in some cases. In Figure 2, Panel B, middle, the Lomb spectrum of a post-cocaine epoch shows a number of spectral peaks with periods
ranging from 3.7 to 6.6 s centered on a powerful main peak of 4.9 s. Inspection of the corresponding scalogram reveals that the period of the neuron's strong oscillatory activity was
continuously shifting within the analyzed time, with an overall decrease in period. Hence, in this case the multiple peaks in the Lomb spectrum arise from the period variation of the
predominant oscillation over time. Alternatively, concerning the weaker oscillatory activity present in Figure 2, Panel B, right, the Lomb periodogram indicates three spectral peaks of
nearly equal strength, at 7.7, 14, and 33 s. The scalogram demonstrates that the 33 s oscillation was present throughout the analyzed segment, simultaneously with the 7.7 and 14 s
oscillations, which themselves were segregated to early and later parts of the segment. Therefore, the presence of multiple spectral peaks can indicate simultaneous oscillations and/or
shifts in oscillation period over time. Periodic oscillations in activity in the seconds-to-minutes range have been noted in many biological systems, and have been attributed with various
functions. In particular, oscillatory fluctuations in intracellular levels of the second-messenger calcium are more efficient than simple changes in mean calcium level for inducing gene
expression, enzymatic activity and hormone secretion in some _in vitro_ systems (Holl et al. 1988; Matozaki et al. 1990; Dolmetsch et al. 1998; De Koninck and Schulman 1998; Li et al. 1998;
Charles et al. 1999; Narenjkar et al. 1999; Tomić et al. 1999). Furthermore, it was hypothesized in some of these studies that the calcium-mediated influence on effector systems is related
to oscillatory frequency. The oscillations in neuronal firing rate in the present study presumably drive slow variations in intracellular calcium concentration which might be functionally
significant; however, they are likely to have effects beyond signal transduction, namely at systems and behavioral levels. Such prominent temporal patterning in basal ganglia spiking
activity is likely to be associated with corresponding patterning in activity of other forebrain regions (_via_ the basal ganglia ouput pathways), and so to modulation of cognition and
behavior. Cocaine and GBR-12909 (as well as D-amphetamine and methylphenidate) induce stereotyped sniffing and oral movements, stereotyped locomotion, and perseveration during the execution
of learned motor behaviors (Kokkinidis and Anisman 1976; Ridley et al. 1981; Mueller et al. 1989; Paulus et al. 1993; Rosenzweig-Lipson et al. 1994; present results) in rodents and non-human
primates. Cocaine or amphetamine intoxication in humans can lead to similar behaviors (reviewed in Lyon and Robbins 1975). GP neurons are well-characterized as having alterations in firing
rate in relation to movement, so that multisecond oscillations in pallidal firing rate might relate to some specific periodicity of limb or body movement, or motor state. Such relationships
will need to be characterized in studies of freely-moving animals. In addition to motor effects, cocaine, amphetamine and methylphenidate all produce a ‘high’ or ‘rush’ at sufficient doses
in humans, and continued usage typically leads to addiction. In studies of rodents and non-human primates, all these stimulants as well as GBR-12909 (Bergman et al. 1989; Roberts 1993)
support self-administration. The similarity of the effects of these drugs on basal ganglia multisecond oscillations, and their common effects on these behaviors, suggests that abnormal
multisecond patterning in the forebrain (with shifted frequency range and/or increased power) might relate to the acute motor and affective/cognitive effects of stimulant treatment, and
might represent a state of brain activity which induces or augments addictive processes. REFERENCES * Allers KA, Kreiss DS, Twery MJ, Juncos JL, Walters JR . (1996): The role of glutamate in
D1 agonist induced increases of subthalamic nucleus neuronal firing rate: normal vs. 6-OHDA lesioned rats. _Soc Neurosci Abstr_ 22: 1200 Google Scholar * Allers KA, Kreiss DS, Walters JR .
(2000): Multisecond oscillations in the subthalamic nucleus: effects of apomorphine and dopamine cell lesion. _Synapse_ 38: 38–50 Article CAS Google Scholar * Allers KA, Ruskin DN,
Bergstrom DA, Molnar LR, Walters JR . (1999): Correlations of multisecond oscillations in firing rate in pairs of basal ganglia neurons. _Soc Neurosci Abstr_ 25: 1929 Google Scholar * Baek
D, Ruskin DN, Bergstrom DA, Walters JR . (1999): D-amphetamine (AMPH) and cocaine increase the speed of multisecond oscillations in globus pallidus (GP) unit activity. _Soc Neurosci Abstr_
25: 1819 Google Scholar * Barbieri EJ, Ferko AP, DeGregorio GJ, Ruch EK . (1992): The presence of cocaine and benzoylecgonine in rat cerebrospinal fluid after the intravenous administration
of cocaine. _Life Sci_ 51: 1739–1746 Article CAS Google Scholar * Barnes CD, Burnham E . (1969): The reflection of third order blood pressure waves in the lumbar monosynaptic reflex.
_Brain Res_ 13: 183–186 Article CAS Google Scholar * Baumann MH, Char GU, De Costa BR, Rice KC, Rothman RB . (1994): GBR12909 attenuates cocaine-induced activation of mesolimbic dopamine
neurons in the rat. _J Pharmacol Exp Ther_ 271: 1216–1222 CAS PubMed Google Scholar * Bergman J, Madras BK, Johnson SE, Spealman RD . (1989): Effects of cocaine and related drugs in
nonhuman primates. III. Self-administration by squirrel monkeys. _J Pharmacol Exp Ther_ 251: 150–155 CAS PubMed Google Scholar * Bergstrom DA, Walters JR . (1981): Neuronal responses of
the globus pallidus to systemic administration of _d_-amphetamine: investigation of the involvement of dopamine, norepinephrine, and serotonin. _J Neurosci_ 1: 292–299 Article CAS Google
Scholar * Bertram D, Barrès C, Julien C . (1999): Effect of desipramine on spontaneous arterial pressure oscillations in the rat. _Eur J Pharmacol_ 378: 265–271 Article CAS Google Scholar
* Beurrier C, Congar P, Bioulac B, Hammond C . (1999): Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. _J Neurosci_ 19: 599–609 Article CAS Google
Scholar * Charles AC, Piros ET, Evans CJ, Hales TG . (1999): L-type Ca2+ channels and K+ channels specifically modulate the frequency and amplitude of spontaneous Ca2+ oscillations and have
distinct roles in prolactin release in GH3 cells. _J Biol Chem_ 274: 7508–7515 Article CAS Google Scholar * Church WH, Justice JB Jr, Byrd LD . (1987): Extracellular dopamine in rat
striatum following uptake inhibition by cocaine, nomifensine and benztropine. _Eur J Pharmacol_ 139: 345–348 Article CAS Google Scholar * De Koninck P, Schulman H . (1998): Sensitivity of
CaM kinase II to the frequency of Ca2+ oscillations. _Science_ 279: 227–230 Article CAS Google Scholar * Dolmetsch RE, Xu K, Lewis RS . (1998): Calcium oscillations increase the
efficiency and specificity of gene expression. _Nature_ 392: 933–936 Article CAS Google Scholar * Engberg G, Elverfors A, Jonason J, Nissbrandt H . (1997): Inhibition of dopamine
re-uptake: significance for nigral dopamine neuron activity. _Synapse_ 25: 215–226 Article CAS Google Scholar * Fink-Jensen A, Ingwersen SH, Nielsen PG, Hansen L, Nielsen EB, Hansen AJ .
(1994): Halothane anesthesia enhances the effect of dopamine uptake inhibition on interstitial levels of striatal dopamine. _Naunyn Schmeidebergs Arch Pharmacol_ 350: 239–244 Article CAS
Google Scholar * Fox SH, Brotchie JM . (1996): Normethylclozapine potentiates the action of quinpirole in the 6-hydroxydopamine lesioned rat. _Eur J Pharmacol_ 301: 27–30 Article CAS
Google Scholar * Fox SH, Brotchie JM . (2000): 5-HT2C receptor antagonists enhance the behavioural response to dopamine D1 receptor agonists in the 6-hydroxydopamine-lesioned rat. _Eur J
Pharmacol_ 398: 59–64 Article CAS Google Scholar * Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG . (1999): Role of serotonin in the paradoxical calming effects of
psychostimulants on hyperactivity. _Science_ 283: 397–401 Article CAS Google Scholar * Grabowska M . (1976): The involvement of serotonin in the mechanism of central action of
apomorphine. _Pol J Pharmacol Pharm_ 28: 389–394 Article CAS Google Scholar * Henry B, Crossman AR, Brotchie JM . (1998): Characterization of enhanced behavioral responses to L-DOPA
following repeated administration in the 6-hydroxydopamine-lesioned rat model of Parkinson's disease. _Exp Neurol_ 151: 334–342 Article CAS Google Scholar * Holl RW, Thorner MO,
Mandell GL, Sullivan JA, Sinha YM, Leong DA . (1988): Spontaneous oscillations of intracellular calcium and growth hormone secretion. _J Biol Chem_ 263: 9682–9685 CAS PubMed Google Scholar
* Huang K-X, Bergstrom DA, Ruskin DN, Walters JR . (1998): N-methyl-D-aspartate receptor blockade attenuates D1 dopamine receptor modulation of neuronal activity in rat substantia nigra.
_Synapse_ 30: 18–29 Article CAS Google Scholar * Kaneoke Y, Vitek JL . (1996): Burst and oscillation as disparate neuronal properties. _J Neurosci Meth_ 68: 211–223 Article CAS Google
Scholar * Kelland MD, Soltis RP, Anderson LA, Bergstrom DA, Walters JR . (1995): In vivo characterization of two cell types in the rat globus pallidus which have opposite responses to
dopamine receptor stimulation: comparison of electrophysiological properties and responses to apomorphine, dizocilpine, and ketamine anesthesia. _Synapse_ 20: 338–350 Article CAS Google
Scholar * Kelland Sr MD, Walters JR . (1992): Apomorphine-induced changes in striatal and pallidal neuronal activity are modified by NMDA and muscarinic receptor blockade. _Life Sci_ 50:
PL179–PL184 Article CAS Google Scholar * Kokkinidis L, Anisman H . (1976): Interaction between cholinergic and catecholaminergic agents in a spontaneous alternation task.
_Psychopharmacology_ 48: 261–270 Article CAS Google Scholar * Koshiya N, Smith JC . (1999): Neuronal pacemaker for breathing visualized _in vitro_. _Nature_ 400: 360–363 Article CAS
Google Scholar * Leresche N, Lightowler S, Soltesz I, Jassik-Gerschenfeld D, Crunelli V . (1991): Low-frequency oscillatory activities intrinsic to rat and cat thalamocortical cells. _J
Physiol_ 441: 155–174 Article CAS Google Scholar * Lewalle J, Peek FW, Murphy SJ . (1995): Wavelet analysis of olfactory nerve response to stimulus. _J Theor Biol_ 177: 215–236 Article
CAS Google Scholar * Li W-h, Llopis J, Whitney M, Zlokarnik G, Tsien RY . (1998): Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. _Nature_
392: 936–941 Article CAS Google Scholar * Lyon M, Robbins T . (1975): The action of central nervous system stimulant drugs: a general theory concerning amphetamine effects. In Essman WB,
Valzelli L (eds), _Current Developments in Psychopharmacology_, Vol. 2. New York, Spectrum Publications, Inc., pp 80–163 Google Scholar * Mabry PD, Campbell BA . (1973): Serotonergic
inhibition of catecholamine-induced behavioral arousal. _Brain Res_ 49: 381–391 Article CAS Google Scholar * Matozaki T, Göke B, Tsunoda Y, Rodriguez M, Martinez J, Williams JA . (1990):
Two functionally distinct cholecystokinin receptors show different modes of actions on Ca2+ mobilization and phospholipid hydrolysis in isolated rat pancreatic acini. _J Biol Chem_ 265:
6247–6254 CAS PubMed Google Scholar * Mautner-Huppert D, Haberl RL, Dirnagl U, Villringer A, Schmiedek P, Einhäupl K . (1989): B-waves in healthy persons. _Neurol Res_ 11: 194–196 Article
CAS Google Scholar * Melis M, Mereu G, Lilliu V, Quartu M, Diana M, Gessa GL . (1998): Haloperidol does not produce dopamine cell depolarization-block in paralyzed, unanesthetized rats.
_Brain Res_ 783: 127–132 Article CAS Google Scholar * Mueller K, Hollingsworth EM, Cross DR . (1989): Another look at amphetamine-induced stereotyped locomotor activity in rats using a
new statistic to measure locomotor stereotypy. _Psychopharmacology_ 97: 74–79 Article CAS Google Scholar * Narenjkar J, Marsh SJ, Assem ESK . (1999): The characterization and
quantification of antigen-induced Ca2+ oscillations in a rat basophilic leukaemia cell line (RBL-2H3). _Cell Calcium_ 26: 261–269 Article CAS Google Scholar * Paulus MP, Callaway CW,
Geyer MA . (1993): Quantitative assessment of the microstructure of rat behavior: II. Distinctive effects of dopamine releasers and uptake inhibitors. _Psychopharmacology_ 113: 187–198
Article CAS Google Scholar * Plenz D, Kitai ST . (1999): A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. _Nature_ 400: 677–682 Article CAS
Google Scholar * Przybyszewski AW . (1991): An analysis of the oscillatory patterns in the central nervous system with the wavelet method. _J Neurosci Meth_ 38: 247–257 Article CAS Google
Scholar * Ridley RM, Baker HF, Haystead TA . (1981): Perseverative behaviour after amphetamine; dissociation of response tendency from reward association. _Psychopharmacology_ 75: 283–286
Article CAS Google Scholar * Roberts DCS . (1993): Self-administration of GBR-12909 on a fixed-ratio and progressive ratio schedule in rats. _Psychopharmacology_ 111: 202–206 Article CAS
Google Scholar * Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crabbe JC, Nestler EJ, Hen R . (1998): Increased vulnerability to cocaine in mice lacking the serotonin-1B
receptor. _Nature_ 393: 175–178 Article CAS Google Scholar * Rosenzweig-Lipson S, Hesterberg P, Bergman J . (1994): Observational studies of dopamine D1 and D2 agonists in squirrel
monkeys. _Psychopharmacology_ 116: 9–18 Article CAS Google Scholar * Ruskin DN, Bergstrom DA, Kaneoke Y, Patel BN, Twery MJ, Walters JR . (1999a): Multisecond oscillations in firing rate
in the basal ganglia: Robust modulation by dopamine receptor activation and anesthesia. _J Neurophysiol_ 81: 2046–2055 Article CAS Google Scholar * Ruskin DN, Bergstrom DA, Shenker A,
Freeman LE, Baek D, Walters JR . (2001): Drugs used in the treatment of attention-deficit/hyperactivity disorder affect postsynaptic firing rate and oscillation without preferential dopamine
autoreceptor action. _Biol Psychiatry_, 49: 340–350 Article CAS Google Scholar * Ruskin DN, Bergstrom DA, Walters JR . (1999b): Firing rates and multisecond oscillations in the rodent
entopeduncular nucleus (EPN): effects of dopamine (DA) agonists and nigrostriatal lesion. _Soc Neurosci Abstr_ 25: 1929 Google Scholar * Ruskin DN, Bergstrom DA, Walters JR . (1999c):
Multisecond oscillations in firing rate in the globus pallidus: synergistic modulation by D1 and D2 dopamine receptors. _J Pharmacol Exp Ther_ 290: 1493–1501 CAS PubMed Google Scholar *
Ruskin DN, Bergstrom DA, Walters JR . (2000): Spatially distant neurons in the basal ganglia can demonstrate correlated multisecond periodicities in firing rate _in vivo_. Winter Conference
on Brain Research; Breckenridge, Colorado. * Ruskin DN, Rawji SS, Walters JR . (1998): Effects of full D1 dopamine receptor agonists on firing rates in the globus pallidus and substantia
nigra pars compacta _in vivo_: tests for D1 receptor selectivity and comparisons in the partial agonist SKF 38393. _J Pharmacol Exp Ther_ 286: 272–281 CAS PubMed Google Scholar * Tomić M,
Koshimizu T-a, Yuan D, Andric SA, Zivadinovic D, Stojilkovic SS . (1999): Characterization of a plasma membrane calcium oscillator in rat pituitary somatotrophs. _J Biol Chem_ 274:
35693–35702 Article Google Scholar * Ungerstedt U . (1971): Striatal dopamine release after amphetamine or nerve degeneration revealed by rotational behavior. _Acta Physiol Scand_ Suppl
367: 49–68 Article CAS Google Scholar * Wichmann T, Bergman H, Kliem MA, Soares J, DeLong MR . (1999): Low-frequency oscillatory discharge in the primate substantia nigra pars reticulata
(SNr) in the normal and parkinsonian state. _Soc Neurosci Abstr_ 25: 1928 Google Scholar * Zouridakis G, Tam DC . (1997): Multi-unit spike discrimination using wavelet transforms. _Comput
Biol Med_ 27: 9–18 Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Experimental Therapeutics Branch, National Institute of Neurological
Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA David N Ruskin Ph.D, Debra A Bergstrom Ph.D, David Baek BS, Lauren E Freeman BS & Judith R Walters Ph.D Authors *
David N Ruskin Ph.D View author publications You can also search for this author inPubMed Google Scholar * Debra A Bergstrom Ph.D View author publications You can also search for this author
inPubMed Google Scholar * David Baek BS View author publications You can also search for this author inPubMed Google Scholar * Lauren E Freeman BS View author publications You can also
search for this author inPubMed Google Scholar * Judith R Walters Ph.D View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR
Correspondence to David N Ruskin Ph.D. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ruskin, D., Bergstrom, D., Baek, D. _et al._ Cocaine or Selective
Block of Dopamine Transporters Influences Multisecond Oscillations in Firing Rate in the Globus Pallidus. _Neuropsychopharmacol_ 25, 28–40 (2001).
https://doi.org/10.1016/S0893-133X(00)00241-4 Download citation * Received: 22 June 2000 * Revised: 22 September 2000 * Accepted: 17 November 2000 * Issue Date: 01 July 2001 * DOI:
https://doi.org/10.1016/S0893-133X(00)00241-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Cocaine * Dopamine * GBR-12909 * Globus pallidus *
NMDA receptor * Oscillation * Wavelet
