Treatment of relapsed b-cell non-hodgkin's lymphoma with a combination of chimeric anti-cd20 monoclonal antibodies (rituximab) and g-csf: final report on safety and efficacy
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Antibody-dependent cellular cytotoxicity (ADCC) is one of the possible mechanisms of action of the chimeric CD20 monoclonal antibody IDEC-C2B8 (rituximab). As granulocyte-colony
stimulating factor (G-CSF) greatly enhances the cytotoxicity of neutrophils in ADCC, the efficacy of rituximab might be enhanced by the addition of G-CSF. In a phase I/II clinical trial, we
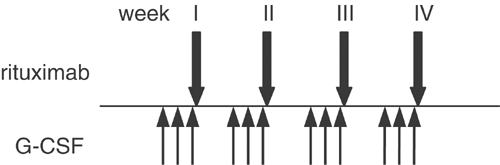
investigated the safety and efficacy of the combination of rituximab and G-CSF (5 μg/kg/day, administered for 3 days, starting 2 days before each infusion) in 26 relapsed low-grade lymphoma
patients. Adverse events occurred in 25/26 patients and mainly consisted of (grade I/II) fever (29%) and allergic reactions (19%). In phases I and II (375 mg/m2 rituximab+G-CSF), 19 patients
were evaluable for efficacy. The response rate was 42% (8/19; 95% CI 20–67%), with 16% (3/19) complete remissions and 26% (5/19) partial remissions. The median duration of response was 18
months, the median time to progression was 24 months. We conclude that the combination of rituximab and G-CSF is well tolerated. Although the overall response rate seems comparable to that
reported for rituximab monotherapy, remission duration in this pilot phase II study is remarkably long. Randomized comparison with rituximab monotherapy should substantiate this promising
finding. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to
this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy
now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS EFFICACY AND SAFETY OF RELMACABTAGENE AUTOLEUCEL, AN ANTI-CD19 CHIMERIC ANTIGEN RECEPTOR T CELL, IN RELAPSED/REFRACTORY B-CELL NON-HODGKIN’S
LYMPHOMA: 2-YEAR RESULTS OF A PHASE 1 TRIAL Article 07 December 2022 EPCORITAMAB INDUCES POTENT ANTI-TUMOR ACTIVITY AGAINST MALIGNANT B-CELLS FROM PATIENTS WITH DLBCL, FL AND MCL,
IRRESPECTIVE OF PRIOR CD20 MONOCLONAL ANTIBODY TREATMENT Article Open access 18 February 2021 CD28-COSTIMULATED CD19 CAR-T CELLS FOR PEDIATRIC MATURE NON-HODGKIN B-CELL LYMPHOMA Article Open
access 23 April 2025 REFERENCES * Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA _et al_. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients
with relapsed low-grade non-Hodgkin's lymphoma. _Blood_ 1997; 90: 2188–2195. CAS PubMed Google Scholar * McLaughlin P, Grillo-López A, Link BK, Levy R, Czuczman MS, Williams ME _et
al_. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. _J Clin Oncol_ 1998; 16: 2825–2833.
Article CAS PubMed Google Scholar * Davis TA, Grillo-Lopez AJ, White CA, McLaughlin P, Czuczman MS, Link BK _et al_. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin's
lymphoma: safety and efficacy of re-treatment. _J Clin Oncol_ 2000; 18: 3135–3143. Article CAS PubMed Google Scholar * Hainsworth JD, Litchy S, Burris HA, Scullin Jr DC, Corso SW,
Yardley DA _et al_. Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin's lymphoma. _J Clin Oncol_ 2002; 20: 4261–4267. Article CAS PubMed Google
Scholar * Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R _et al_. Depletion of B cells _in vivo_ by a chimeric mouse human monoclonal antibody to CD20. _Blood_ 1994; 83:
435–445. CAS PubMed Google Scholar * Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri GM, Bernasconi S _et al_. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody
rituximab _in vitro_: CD55 and CD59 regulate complement-mediated cell lysis. _Blood_ 2000; 95: 3900–3908. CAS PubMed Google Scholar * Harjunpaa A, Junnikkala S, Meri S . Rituximab
(Anti-CD20) therapy of B-cell lymphomas: direct complement killing is superior to cellular effector mechanisms. _Scand J Immunol_ 2000; 51: 634–641. Article CAS PubMed Google Scholar *
Tedder TF, Boyd AW, Freedman AS, Nadler LM, Schlossman SF . The B cell surface molecule B1 is functionally linked with B cell activation and differentiation. _J Immunol_ 1985; 135: 973–979.
CAS PubMed Google Scholar * Hofmeister JK, Cooney D, Coggeshall KM . Clustered CD20 induced apoptosis: Src-family kinase the proximal regulator of tyrosine phosphorylation, calcium
influx, and caspase 3-dependent apoptosis. _Blood Cells Mol Dis_ 2000; 26: 133–143. Article CAS PubMed Google Scholar * Shan D, Ledbetter JA, Press OW . Apoptosis of malignant human B
cells by ligation of CD20 with monoclonal antibodies. _Blood_ 1998; 91: 1644–1652. CAS PubMed Google Scholar * Shan D, Ledbetter JA, Press OW . Signaling events involved in
anti-CD20-induced apoptosis of malignant human B cells. _Cancer Immunol Immunother_ 2000; 48: 673–683. Article CAS PubMed Google Scholar * Clynes RA, Towers TL, Presta LG, Ravetch JV .
Inhibitory Fc receptors modulate _in vivo_ cytoxicity against tumor targets. _Nat Med_ 2000; 6: 443–446. Article CAS PubMed Google Scholar * Cartron G, Dacheux L, Salles G, Solal-Celigny
P, Bardos P, Colombat P _et al_. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. _Blood_ 2002; 99: 754–758. Article
CAS PubMed Google Scholar * Kaminski MS, Zasadny KR, Francis IR, Fenner MC, Ross CW, Milik AW _et al_. Iodine-131-anti-B1 radioimmunotherapy for B-cell lymphoma. _J Clin Oncol_ 1996; 14:
1974–1981. Article CAS PubMed Google Scholar * Liu SY, Eary JF, Petersdorf SH, Martin PJ, Maloney DG, Appelbaum FR _et al_. Follow-up of relapsed B-cell lymphoma patients treated with
iodine-131-labeled anti-CD20 antibody and autologous stem-cell rescue. _J Clin Oncol_ 1998; 16: 3270–3278. Article CAS PubMed Google Scholar * Sacchi S, Federico M, Vitolo U, Boccomini
C, Vallisa D, Baldini L _et al_. Clinical activity and safety of combination immunotherapy with IFN-alpha 2a and Rituximab in patients with relapsed low grade non-Hodgkin's lymphoma.
_Haematologica_ 2001; 86: 951–958. CAS PubMed Google Scholar * Davis TA, Maloney DG, Grillo-Lopez AJ, White CA, Williams ME, Weiner GJ _et al_. Combination immunotherapy of relapsed or
refractory low-grade or follicular non-Hodgkin's lymphoma with rituximab and interferon-alpha-2a. _Clin Cancer Res_ 2000; 6: 2644–2652. CAS PubMed Google Scholar * Valerius T, Repp
R, de Wit T, Berthold S, Platzer E, Kalden JR _et al_. Involvement of the high-affinity receptor for IgG (Fc gamma RI; CD64) in enhanced tumor cell cytotoxicity of neutrophils during
granulocyte colony-stimulating factor therapy. _Blood_ 1993; 82: 931–939. CAS PubMed Google Scholar * Kerst J, de Haas M, van der Schoot C, Slaper-Cortenbach I, Kleijer M, von dem Borne A
_et al_. Recombinant granulocyte colony-stimulating factor administration to healthy volunteers: induction of immunophenotypically and functionally altered neutrophils via an effect on
myeloid progenitor cells. _Blood_ 1993; 82: 3265–3272. CAS PubMed Google Scholar * Elsasser D, Valerius T, Repp R, Weiner GJ, Deo Y, Kalden JR _et al_. HLA class II as potential target
antigen on malignant B cells for therapy with bispecific antibodies in combination with granulocyte colony-stimulating factor. _Blood_ 1996; 87: 3803–3812. CAS PubMed Google Scholar *
Repp R, Valerius T, Sendler A, Gramatzki M, Iro H, Kalden JR _et al_. Neutrophils express the high affinity receptor for IgG (Fc gamma RI, CD64) after _in vivo_ application of recombinant
human granulocyte colony-stimulating factor. _Blood_ 1991; 78: 885–889. CAS PubMed Google Scholar * IDEC Pharmaceuticals Corporation. Investigational Drug Brochure Rituximab (RituxanTM,
Mab Thera, IDEC-C2B8), 4th edn. San Diego, CA, USA: IDEC Pharmaceuticals Corporation, 1997. * Nuijens JH, Abbink JJ, Wachtfogel YT, Colman RW, Eerenberg AJ, Dors D _et al_. Plasma elastase
alpha 1-antitrypsin and lactoferrin in sepsis: evidence for neutrophils as mediators in fatal sepsis. _J Lab Clin Med_ 1992; 119: 159–168. CAS PubMed Google Scholar * de Haas M, Kerst JM,
van der Schoot C, Calafat J, Hack CE, Nuijens JH _et al_. Granulocyte colony-stimulating factor administration to healthy volunteers: analysis of the immediate activating effects on
circulating neutrophils. _Blood_ 1994; 84: 3885–3894. CAS PubMed Google Scholar * Foran JM, Gupta RK, Cunningham D, Popescu RA, Goldstone AH, Sweetenham JW _et al_. A UK multicentre phase
II study of rituximab (chimaeric anti-CD20 monoclonal antibody) in patients with follicular lymphoma, with PCR monitoring of molecular response. _Br J Haematol_ 2000; 109: 81–88. Article
CAS PubMed Google Scholar * Lieschke GJ, Burgess AW . Granulocyte colony-stimulating factor and granulocyte–macrophage colony-stimulating factor (1). _N Engl J Med_ 1992; 327: 28–35.
Article CAS PubMed Google Scholar * Maloney DG, Grillo-Lopez AJ, Bodkin DJ, White CA, Liles TM, Royston I, Varns C _et al_. IDEC-C2B8: results of a phase I multiple-dose trial in
patients with relapsed non-Hodgkin's lymphoma. _J Clin Oncol_ 1997; 15: 3266–3274. Article CAS PubMed Google Scholar * van der Kolk LE, de Haas M, Grillo-Lopez AJ, Baars JW, van
Oers MH . Analysis of CD20-dependent cellular cytotoxicity by G-CSF-stimulated neutrophils. _Leukemia_ 2002; 16: 693–699. Article CAS PubMed Google Scholar * Weng K-W, Levy R . Analysis
of IgG Fc Receptor FcgRIIIa polymorphism in relapsed follicular non-hodgkin's lymphoma patients treated with rituximab [abstract]. _Blood_ 2002; 100: 1368a. Google Scholar * Maloney
DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A _et al_. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody
(IDEC-C2B8) in patients with recurrent B-cell lymphoma. _Blood_ 1994; 84: 2457–2466. CAS PubMed Google Scholar * Leavey PJ, Sellins KS, Thurman G, Elzi D, Hiester A, Silliman CC _et al_.
_In vivo_ treatment with granulocyte colony-stimulating factor results in divergent effects on neutrophil functions measured _in vitro_. _Blood_ 1998; 92: 4366–4374. CAS PubMed Google
Scholar * Price TH, Chatta GS, Dale DC . Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. _Blood_ 1996; 88: 335–340.
CAS PubMed Google Scholar * Hoglund M, Hakansson L, Venge P . Effects of _in vivo_ administration of G-CSF on neutrophil functions in healthy volunteers. _Eur J Haematol_ 1997; 58:
195–202. Article CAS PubMed Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Hematology, Academic Medical Center, Amsterdam, The Netherlands
L E van der Kolk & M H J van Oers * IDEC Pharmaceuticals Corp., San Diego, CA, USA A J Grillo-López * Department of Medical Oncology, Antoni van Leeuwenhoek Hospital/Netherlands Cancer
Institute, Amsterdam, The Netherlands J W Baars Authors * L E van der Kolk View author publications You can also search for this author inPubMed Google Scholar * A J Grillo-López View author
publications You can also search for this author inPubMed Google Scholar * J W Baars View author publications You can also search for this author inPubMed Google Scholar * M H J van Oers
View author publications You can also search for this author inPubMed Google Scholar RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE van der Kolk, L.,
Grillo-López, A., Baars, J. _et al._ Treatment of relapsed B-cell non-Hodgkin's lymphoma with a combination of chimeric anti-CD20 monoclonal antibodies (rituximab) and G-CSF: final
report on safety and efficacy. _Leukemia_ 17, 1658–1664 (2003). https://doi.org/10.1038/sj.leu.2402995 Download citation * Received: 25 June 2002 * Accepted: 25 March 2003 * Published: 29
July 2003 * Issue Date: 01 August 2003 * DOI: https://doi.org/10.1038/sj.leu.2402995 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * rituximab
* G-CSF * low-grade B-cell lymphoma * clinical trial * ADCC
