Restoration of frequency-dependent depression of the H-reflex by passive exercise in spinal rats
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Hyper-reflexia, measured as a decrease of low frequency-dependent depression of the H-reflex, is known to occur in both humans and animals after spinal cord injury (SCI). Previous studies
have shown that passive exercise for 3 months could be used to restore low frequency-dependent depression of the H-reflex after SCI.
To determine the effects of various periods of time on the ability of passive exercise to restore low frequency-dependent depression of the H-reflex.
Spinal Cord Injury Mobilization Program of the Center for Translational Neuroscience, the research arm of the Jackson T Stephens Spine & Neuroscience Institute, Little Rock, AR, USA.
Adult rats underwent complete spinal cord transection at the T10 level. The hindlimbs were passively exercised in different groups of rats for 1 h/day, 5 days/week for 15, 30, 45, 60, or 90
days, and low frequency-dependent depression of the H-reflex was tested.
Statistically significant low frequency-dependent depression of the H-reflex was evident by 30 days of exercise, although numerical reductions were seen even at 15 days. There was a linear
decrease in low frequency-dependent depression of the H-reflex with duration of passive exercise.
Passive exercise can restore frequency-dependent depression of spinal reflexes in a time-dependent manner if used following complete spinal transection.
Supported by USPHS award RR020146 and the Arkansas Biosciences Institute.
Spinal cord injury (SCI) results in numerous deficits of the motor and sensory systems, including paralysis, anesthesia, and hyper-reflexia below the level of the lesion. Hyper-reflexia is
evident in both humans and animals following SCI. The physiological changes that have been postulated to contribute to hyper-reflexia include alpha motoneuron hyperexcitability,1, 2, 3
changes in the intrinsic properties of alpha motoneurons,4, 5, 6, 7 reduced post-activation depression of transmission from Ia fibers,8, 9 synapse growth;10 alterations in morphology of
alpha motoneurons,11 and decreased presynaptic inhibition of Ia terminals.9, 12, 13, 14, 15 The time course of spinal changes after injury has been proposed to include an early postsynaptic
mechanism, possibly involving an increase in excitability and/or receptor upregulation, and a late change involving presynaptic mechanisms possibly involving synaptic growth in spared
descending pathways and in reflex pathways.10 One measure used by numerous investigators to quantify hyper-reflexia is the electrical analogue of the classic tendon jerk reflex, referred to
as the Hoffman or H-reflex.2, 15, 16, 17, 18, 19 The H-reflex is a compound electromyographic (EMG) response elicited by the synaptic activation of motoneurons by muscle afferents following
stimulation of muscle nerves. Thompson et al20 investigated four measures of H-reflex excitability in a contusion model of SCI in the rat. Results of their studies led these researchers to
conclude that rate-sensitive depression of the H-reflex was of particular importance in the assessment of hyper-reflexia following SCI. Other groups have reached similar conclusions
regarding the importance of changes in H-reflex rate-sensitive depression as a measure of the effects of SCI.21 In spinally intact individuals, the H-reflex demonstrates depressed amplitude,
due to marked frequency-dependent depression, once stimulus frequencies reach or exceed 1 Hz.22, 23 However, frequency-dependent depression of the H-reflex is less evident in patients or
animals with chronic SCI.12, 13, 22, 24, 25 Previously, we reported the ability of long-term passive exercise therapy to restore frequency-dependent depression of the H-reflex in adult rats
with complete spinal cord transections.24, 25 Specifically, we found that a period of 3 months of motorized bicycle exercise training (MBET), performed in episodes of 1 h/day, 5 days/week,
was capable of restoring frequency-dependent depression of the H-reflex to the level of intact animals.24, 25 The current studies were undertaken to determine the time course of this
decrease in hyper-reflexia, and if shorter periods of total exercise are capable of producing similar restoration of H-reflex frequency-dependent depression in adult rats following spinal
cord transection. Preliminary results have been reported.25
Adult female Sprague–Dawley rats (Harlan, 200–250 g, n=40) underwent a lower thoracic laminectomy under ketamine (60 mg/kg, i.m.) and xylazine (10 mg/kg, i.m.) anesthesia. A complete
transection (Tx) of the spinal cord was made by aspiration and the transected ends of the cord retracted, producing a 2–3 mm cavity. Gelfoam was inserted into the cavity to facilitate
hemostasis and the dura was closed over the Tx site. Muscle and skin were sutured in separate layers, and animals were provided with dextrose–saline (5%, 1 ml/100 g body weight, s.c.) to
replace fluid lost during the surgical procedure. Penicillin (5000 U, i.m.) was administered immediately postoperatively, and animals were transferred to an incubator maintained at 37.5°C
until fully recovered from the anesthetic. The urinary bladder of each animal was expressed manually twice daily until reflexive voiding was established (10–14 days). Animals were monitored
for signs of urinary tract infection, and treatment with Baytril (enroflaxin 0.2 mg/day, i.m. for 10 days) was instituted as needed. All procedures were approved by the Institutional Animal
Use and Care Committee at UAMS.
One group of rats (Tx only 90D, n=4) underwent no further treatment until reflex testing was carried out 90 days after Tx, while a second group of transected animals (Tx only 30D, n=8)
underwent no training but was tested for H-reflex frequency-dependent depression after 30 days, and a group of intact rats served as nontransected controls (CONTROL, n=5). The remaining rats
(n=23) were divided into five groups that received MBET daily. Exercise was provided for either 15 days (Tx+Ex 15D, n=5), 30 days (Tx+Ex 30D, n=4), 45 days (Tx+Ex 45D, n=5), 60 days (Tx+Ex
60D, n=5), or 90 days (Tx+Ex 90D, n=4). The exercise regimen MBET involved suspending the rats on a sling with the hindlimbs hanging down and the hind paws strapped to the pedals of a
bicycle-type device, which was driven by a motor. The pedaling motion flexed one hindlimb and simultaneously extended the contralateral one, while avoiding overstretching of either limb.
Cycling speed was 30 rpm. Exercise sessions consisted of two 30-min episodes with 10 min of rest in between. After the end of the training period, reflex testing was performed. The two Tx
only groups also underwent reflex testing 30 days or 90 days after Tx, along with a group of intact rats (CTL), for comparison with the MBET experimental groups.
Animals were anesthetized with ketamine (60 mg/kg, i.m.) and maintained with 10% doses as needed such that vibrissal and pinna pinch reflexes were absent. Core body temperature was
maintained at 36±1°C using a thermostatically controlled heat lamp. A bipolar cuff electrode was placed on the tibial nerve for stimulation (0.1 ms pulses, cathode proximal on nerve).
Exposed tissue was covered with mineral oil to prevent drying. A wire electrode was inserted subcutaneously in the digital interosseous muscles between the fourth and fifth metatarsals for
EMG recording as previously demonstrated,26, 27 and referenced to a clip applied to the skin on the digits. A ground electrode was attached to the skin of the tail. Recordings were made
using amplifier (Grass P511) filter settings of 3 Hz to 3 kHz with the 60 Hz notch filter in use. Responses to the stimulus were digitized and averaged using a GW Instruments (Somerville,
MA, USA) digitizer module and SuperScopeR software.
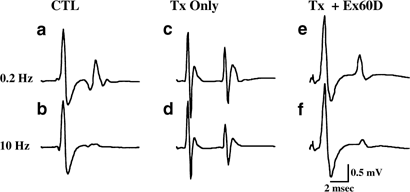
Stimulation of the tibial nerve under the calcaneal tendon produced two responses, an early M-wave (∼2 ms latency), produced by direct activation of motoneuronal axons in the tibial nerve,
and a later H-reflex (∼8 ms latency), produced by activation of muscle afferents in the tibial nerve, which synapse monosynaptically on plantar motoneurons. The degree of stimulation that
induced frequency-dependent depression of the H-reflex was determined. The reflex was first tested at 0.2 Hz to determine threshold and maximal response levels. After discarding the first
five responses in order to obtain an average of the stabilized reflex, averages of 10 responses were obtained. Averages were compiled following stimulation at 0.2, 1, 5, and 10 Hz. The
change in the response at various frequencies was calculated as the percent of the response at 0.2 Hz in order to determine depression of the H-reflex as a function of stimulation frequency.
Following the frequency series testing, the H-reflex amplitude was confirmed at 0.2 Hz for consistency. If the amplitude at recheck was less than 90% of the initial amplitude, the data was
discarded.
At the end of the experiment, animals were euthanized with an overdose of barbiturate (Nembutal) and the Tx was confirmed either visually or histologically following transcardial perfusion
with paraformaldehyde (4%) and sucrose (20%). Sensory testing or assessment of spasticity was not carried out in this series of animals.
The amplitude of the H-wave was measured from the base line before the H-wave to the peak of the first (and largest) of its two components (Figure 1). Measurements from peak to peak of the
two components gave similar results so that only the baseline to peak measures are reported here. Results from animal groups were compared statistically using a two-way ANOVA test.
Significant differences between groups were tested using the Scheffe test, a conservative post hoc comparison. Statistical significance was considered to be present at P
