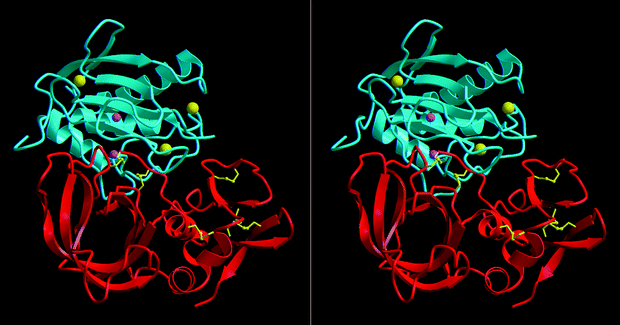
Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by timp-1
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Access through your institution Buy or subscribe Matrix metalloproteinases (MMPs) are zinc endopeptidases that are required for the degradation of extracellular matrix components during
normal embryo development, morphogenesis and tissue remodelling1. Their proteolytic activities are precisely regulated by endogenous tissue inhibitors of metalloproteinases (TIMPs)1,2,3,4,5.
Disruption of this balance results in diseases such as arthritis, atherosclerosis, tumour growth and metastasis1,2. Here we report the crystal structure of an MMP-TIMP complex formed
between the catalytic domain of human stromelysin-1 (MMP-3) and human TIMP-1. TIMP-1, a 184-residue protein5, has the shape of an elongated, contiguous wedge. With its long edge, consisting
of five different chain regions, it occupies the entire length of theactive-site cleft of MMP-3. The central disulphide-linked segments Cys 1-Thr 2-Cys 3-Val 4 and Ser 68-Val 69 bind to
either side of the catalytic zinc. Cys 1 bidentally coordinates this zinc, and the Thr-2 side chain extends into the large specificity pocket of MMP-3. This unusual architecture of the
interface between MMP-3 and TIMP-1 suggests new possibilities for designing TIMP variants and synthetic MMP inhibitors with potential therapeutic applications. The TIMP family at present
comprises four members (TIMP-1 to TIMP-4) of relative molecular mass (_M_r) ranging from 22K to 30K, with 40–50% sequence identity3. All TIMPs inhibit active MMPswith relatively low
selectivity, forming tight non-covalent 1:1 complexes4. Native TIMP-1 contains two glycosylation sites5. AC-terminally truncated form of TIMP-1 comprising only the N-terminal two-thirds of
its polypeptide chain (N-TIMP-1) has been shown to inhibit MMP-3 with a slightly reduced affinity compared with full-length TIMP-1 (refs 4,6,7). These experiments suggested that TIMPs
interact with MMPs predominantly through their N-terminal moiety. A preliminary nuclear magnetic resonance (NMR) model of N-TIMP-2 has been reported8, but the mechanism of MMP inhibition by
the TIMPs is not understood. Most MMPs, including stromelysin-1, are secreted as multimodular proenzymes that can be activated, with a 165-residue catalytic domain flanked by an N-terminal
activation peptide and a spacer-linked C-terminal domain, respectively. Three-dimensional structures have been determined for a few catalytic MMP domains, including those of stromelysin-1
(MMP-3)9,10,11 and its proform10, and for full-length porcine MMP-1 (ref. 12). To understand the mechanism of interaction between MMPs and TIMPs, and to allow the design of morespecific TIMP
species, we have undertaken an X-ray crystal-structure analysis of the complex formed between unglycosylated human TIMP-1 and the catalytic domain of MMP-3. This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only
$3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support REFERENCES * Nagase, H. in _Zinc Metalloproteases in Health and
Disease_(ed. Hooper, N. M.) 153–204 (Taylor and Francis, London, (1996)). Google Scholar * Coussens, L. M. & Werb, Z. Matrix metalloproteinases and the development of cancer. _Chem.
Biol._ 3, 895–904 (1996). Google Scholar * Green, J._et al_. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. _J. Biol. Chem._ 271, 30375–30380
(1996). Google Scholar * Willenbrock, F. & Murphy, G. Structure–function relationships in the tissue inhibitors of metalloproteinases. _Am. J. Resp. Crit. Care Med._ 150, 5165–5170
(1994). Google Scholar * Docherty, A. J. P._et al_. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. _Nature_ 318, 66–69 (1985).
Article ADS CAS Google Scholar * Murphy, G._et al_. The N-terminal domain of human tissue inhibitor of metalloproteinases retains metalloproteinase inhibitory activity. _Biochemistry_
30, 8097–8102 (1991). Google Scholar * 7. Huang, W._et al_. Folding and characterization of the amino-terminal domain of human tissue inhibitor of metalloproteinase-1 (TIMP-1) expressed at
high yield in _E. coli_. _FEBS Lett._ 384, 155–161 (1996). Google Scholar * Williamson, R. A._et al_. Solution structure of the active domain of tissue inhibitor of metalloproteinases-2. A
new member of the OB fold protein family. _Biochemistry_ 33, 11745–11759 (1994). Google Scholar * Gooley, P. R._et al_. NMR structure of inhibited catalytic domain of human stomelysin-1.
_Nature Struct. Biol._ 1, 111–118 (1994). Google Scholar * Becker, J. W._et al_. Stromelysin-1: Three-dimensional structure of the inhibited catalytic domain and of the C-truncated
proenzyme. _Prot. Sci._ 4, 1966–1976 (1995). Google Scholar * Dhanaraj, V._et al_. X-ray structure of a hydroxamate inhibitor complex of stromelysin catalytic domain and its comparison with
members of the zinc metalloproteinase superfamily. _Structure_ 4, 375–386 (1996). Google Scholar * Li, J. -Y._et al_. Structure of full-length porcine synovial collagenase reveals a
C-terminal domain containing a calcium-linked, four-bladed β-propeller. _Structure_ 3, 541–549 (1995). MATH Google Scholar * Murzin, A. G. OB-fold: common structural and functional
solution for non-homologous sequences. _EMBO J._ 12, 861–867 (1993). Google Scholar * Grams, F._et al_. X-ray structures of human neutrophil collagenase complexed with peptide hydroxamate
and peptide thiol inhibitors. Implications for substrate binding and rational drug design. _Eur. J. Biochem._ 228, 830–841 (1995). Google Scholar * Will, H., Atkinson, S. J., Butler, G. S.,
Smyth, B. & Murphy, G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autocatalytic activation. _J.
Biol. Chem._ 271, 17119–17123 (1996). Google Scholar * Huovila, A. P., Ilmeida, E. A. C. & White, J. M. ADAMs and cell fusion. _Curr. Opin. Cell Biol._ 8, 692–699 (1996). Google Scholar
* Stöcker, W._et al_. The metzincins: Topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of
zinc-peptidases. _Protein Sci._ 4, 823–840 (1995). Google Scholar * Kessler, E., Takahara, K., Biniaminov, L., Brusel, M. & Greenspan, D. S. Bone morphogenetic protein-1: The type I
procollagen C-proteinase. _Science_ 271, 360–362 (1996). Google Scholar * Baumann, U., Wu, S., Flaherty, K. M. & McKay, D. B. Three-dimensional structure of the alkaline proteae of
_Pseudomonas aeruginosa_: a two-domain protein with a calcium binding parallel β roll motif. _EMBO J._ 12, 3357–3364 (1993). Google Scholar * Bode, W., Gomis-Rüth, F. X., Huber, R.,
Zwilling, R. & Stöcker, W. Structure of astacin and implications for activation of astacins and zinc-ligation of collagenases. _Nature_ 358, 164–166 (1992). Article ADS CAS Google
Scholar * Gomis-Rüth, F. X., Kress, L. F. & Bode, W. First structure of a snake venom metalloproteinase: prototype for matrix metalloproteinases/collagenases. _EMBO J._ 12, 4151–4157
(1993). Google Scholar * Black, R. A._et al_. Ametalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. _Nature_ 385, 729–733 (1997). Article ADS CAS Google
Scholar * Moss, M. L._et al_. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α. _Nature_ 385, 733–736 (1997). Article ADS CAS Google Scholar
* Otwinowski, Z. & Minor, W. _DENZO: A Film Processing for Macromolecular Crystallography_(Yale University, New Haven, CT, (1993)). Google Scholar * Navaza, J. AMoRe: an automated
package for molecular replacement. _Acta crystallogr. A_ 50, 157–163 (1994). Google Scholar * Otwinowski, Z. in _Isomorphous Replacement and Anomalous Scattering, Daresbury study weekend
proceedings_(SERC Daresbury Laboratory, Warrington, (1991)). Google Scholar * Cowtan, K. _Joint CCP4 ESF-EACBM Newsletter on Protein Crystallography_ VOL. 31(1994). Google Scholar *
Roussel, A. & Cambilleau, C. _Turbo-Frodo in Silicon Graphics Geometry, Partners Directory_(Silicon Graphics, Mountain View, CA, (1989)). Google Scholar * Brünger, A. T.
Crystallographic phasing and refinement of macromolecules. _Curr. Opin. Struct. Biol_ 1, 1016–1022 (1991). Google Scholar * Nicholls, A., Bharadwaj, R. & Honig, B. Grasp–graphical
representation and analysis of surface properties. _Biophys. J._ 64, 166 (1993). Google Scholar Download references ACKNOWLEDGEMENTS We thank M. Braun for help with crystallization, M. T.
Stubbs for reading the manuscript, and A. Lebedev and E. H. Panepucci for help with molecular replacement. This work was supported by the SFB469, the Human Capital and Mobility, and the
Biotechnology programs of the European Union, the Fonds der Chemischen Industrie, the BMBF, and the NIH. AUTHOR INFORMATION Author notes * Franz-Xaver Gomis-R¨th and Klaus Maskos: These
authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Abteilung für Strukturforschung, Max-Planck-Institut für Biochemie, Martinsried, D-82152, Germany Franz-Xaver Gomis-R¨th,
Klaus Maskos, Michael Betz, Andreas Bergner, Robert Huber & Wolfram Bode * Department of Biochemistry and Molecular Biology, University of Kansas Medical Center, Kansas City, 66160,
Kansas, USA Ko Suzuki, Naoki Yoshida & Hideaki Nagase * Department of Biochemistry and Molecular Biology, University of Miami, School of Medicine, Miami, 33101, Florida, USA Keith Brew *
AG Proteindynamik MPG-ASMB, c/o DESY, Hamburg, D-22603, Germany Gleb P. Bourenkov & Hans Bartunik Authors * Franz-Xaver Gomis-R¨th View author publications You can also search for this
author inPubMed Google Scholar * Klaus Maskos View author publications You can also search for this author inPubMed Google Scholar * Michael Betz View author publications You can also search
for this author inPubMed Google Scholar * Andreas Bergner View author publications You can also search for this author inPubMed Google Scholar * Robert Huber View author publications You
can also search for this author inPubMed Google Scholar * Ko Suzuki View author publications You can also search for this author inPubMed Google Scholar * Naoki Yoshida View author
publications You can also search for this author inPubMed Google Scholar * Hideaki Nagase View author publications You can also search for this author inPubMed Google Scholar * Keith Brew
View author publications You can also search for this author inPubMed Google Scholar * Gleb P. Bourenkov View author publications You can also search for this author inPubMed Google Scholar
* Hans Bartunik View author publications You can also search for this author inPubMed Google Scholar * Wolfram Bode View author publications You can also search for this author inPubMed
Google Scholar CORRESPONDING AUTHOR Correspondence to Wolfram Bode. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gomis-R¨th, FX., Maskos, K., Betz, M.
_et al._ Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. _Nature_ 389, 77–81 (1997). https://doi.org/10.1038/37995 Download citation * Received: 30
April 1997 * Accepted: 13 June 1997 * Issue Date: 04 September 1997 * DOI: https://doi.org/10.1038/37995 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
