Increased p53 protein expression in malignant mammary phyllodes tumors
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The authors reviewed 143 cases (87 benign, 37 borderline, and 19 malignant) of mammary phyllodes tumors (PTs) and used immunohistochemistry to detect p53 protein product
semi-quantitatively as negative, weak, moderate and strong (scored 0 to 3). For all PTs, an increasing trend of tumor size and malignancy was detected with increasing age. For p53 staining,
60 cases (42%) were negative, 55 (38%) stained weakly, 28 (13%) stained moderately, and 10 (7%) stained strongly. Of the 87 benign PTs, 41 (47%) were negative, 37 (43%) stained weakly, and 9
(10%) stained moderately. For the 37 borderline PTs, 16 (43%) were negative, 14 (38%) stained weakly, 6 (16%) stained moderately, and 1 (3%) stained strongly. Of the 19 malignant PTs, 3
(16%) were negative, 4 (21%) stained weakly, 3 (16%) stained moderately, and 9 (47%) stained strongly. The mean intensity score for p53 staining increased progressively from benign to
borderline to malignant PT, with established statistical significance (_P_ < .0001). This is significantly correlated with mitotic count but not stromal cellularity, pleomorphism, margin,
and stromal overgrowth. When considering strong staining alone (score, 3), 47% of malignant, 3% of borderline, and none of the benign PTs were positive. The use of strong positive staining
for diagnosing malignant PT gave positive and negative predictive values, specificity, and sensitivity of 90%, 92.5%, 99%, and 47%, respectively. Thus diffuse strong p53 protein staining can
be used as a soft sign in assisting the diagnosis of malignant PT. Conversely, negative or weak staining of p53 protein in PT is of little discriminatory value. The role of p53 gene
mutation in the malignant transformation of PT is unclear; but this may not be the sole mechanism as many malignant PT were p53 protein negative. SIMILAR CONTENT BEING VIEWED BY OTHERS NOVEL
USES OF IMMUNOHISTOCHEMISTRY IN BREAST PATHOLOGY: INTERPRETATION AND PITFALLS Article 27 October 2020 SIGNIFICANCE OF P53 IMMUNOSTAINING IN MESOTHELIAL PROLIFERATIONS AND CORRELATION WITH
_TP53_ MUTATION STATUS Article 08 September 2021 PAPILLARY NEOPLASMS OF THE BREAST—REVIEWING THE SPECTRUM Article 18 January 2021 INTRODUCTION Phyllodes tumor (PT) is an uncommon
stromal–epithelial neoplasm of the breast. The reported incidence is 0.3 to 0.5% of female breast tumors (1, 2, 3, 4, 5). The median and mean age of patients is 45 y, and the average size is
4–5 cm. Rarely, these lesions can occur in younger and older women and in men. Clinically, PT is difficult to distinguish from fibroadenoma. The histological grading of PT is based on a
combination of histological features, including mitotic count, cellularity, and pleomorphism of the stromal cells, stromal overgrowth, and whether the border is infiltrative or not (2, 6).
By using these parameters, PT is divided into benign, borderline, and malignant. Although both borderline and malignant PT can metastasize, all PTs can recur locally. This propensity to
recur makes proper and adequate treatment imperative, even in benign cases. Mutations of the p53 tumor suppressor gene are among the commonest detected in human malignancies (7).
Accumulation of the protein product, as detected by immunohistochemistry, has been described in many tumor types as a marker of neoplastic progression and of aggressiveness (8, 9). In the
literature, evaluation of p53 protein expression in PT has been reported in several studies (10, 11, 12, 13, 14). In the studies that correlated the grade of PT to p53 protein expression, it
was suggested that malignant PT have diffuse strong staining that allowed distinction of malignant from benign and borderline PT (10, 11, 12). Other studies suggested that p53 protein
expression did not predict outcome (11, 13). In the current study, we further evaluated the relationship of p53 protein expression with histologic parameters and the role of p53 protein
detection in the diagnosis of malignancy in PT. MATERIALS AND METHODS The histopathology files from the three participating departments were searched for PT over the past 14 years, yielding
a total of 143 cases. The paraffin blocks were retrieved and 4-μm slides prepared routinely, stained with hematoxylin and eosin. All the slides were reviewed for the following histologic
parameters: (1) stromal cellularity; (2) nuclear pleomorphism; (3) stromal overgrowth; (4) mitotic count; and (5) margin of the tumor, whether infiltrative or rounded. The stromal
cellularity and nuclear pleomorphism were graded as low/mild, moderate, or severe; stromal overgrowth was graded as mild, moderate (scanty epithelial element within a low-power field), or
severe (absence of epithelial element within a low-power field [40 × ]; Nikon Labophot; field area, 1.9 mm2); and the mitotic count was given as the number of mitotic figures per 10
high-power fields (400 ×; Nikon Labophot; field area, 0.19 mm2). A diagnosis of benign PT was made when there was low cellularity, no stromal overgrowth, mild pleomorphism, a rounded margin,
and a mitotic count of two or less per 10 high-power fields. Malignant PT was diagnosed when the mitotic count was five or more per 10 high-power fields, together with stromal overgrowth
and an infiltrative margin. Borderline PT was diagnosed when the criteria for malignancy were not totally fulfilled. For p53 staining, a most-representative slide was taken from each case
and stained for p53 (DO-7 monoclonal, Novocastra, UK) using standard avidin-biotin method with microwave antigen retrieval. The staining of cells was assessed according to both the intensity
and proportion of positive cells. The staining pattern was graded from 0 to 3, with 0 being no staining; 1 when < 33% of the stromal cell nuclei stained weakly; 2 when 34–67% of cell
nuclei stained with weak to moderate staining intensity; and 3 when >67% cells displayed moderate to strong nuclear staining. For statistical analysis, PROC LOGISTIC in SAS was used to
study the association between p53 and diagnosis with the presence of the five histologic factors and recurrence. Student _t_ test and ANOVA was used to compare the tumor size and patient age
with diagnosis of PT and also between the recurrent and nonrecurrent tumors. Statistical significance is established at _P_ < .05. RESULTS One hundred and thirty-seven patients were
included in this study, including four patients with both the initial and first recurrent PT, one patient with the initial and two additional recurrences, six patients with only the first
recurrence, one patient with only the second recurrence, and the initial PT of two patients with one recurrence, yielding a total of 143 PTs. The patient’s age ranged from 15 to 77 years
(mean, 43 y), and the tumor size ranged from 1 to 22 cm (mean, 5 cm). Sixty-two percent of the patients were Chinese, 21% were Caucasian, and the remainder were Indo-Chinese or Indian. The
tumors occurred on the left side in 51% of cases, and 48% occurred on the right; in two cases, the side was not known. Of the total cases, there were 87 (61%) benign, 37 (26%) borderline,
and 19 (13%) malignant PT. For the 87 benign PT, the patient age range was 17 to 62 years (mean, 40.8 y), and the tumor size range was 1 to 22 cm (mean, 4.3 cm). For the 37 borderline PT,
the patient age range was 15 to 77 years (mean, 44.6 y), and the tumor size range was 1 to 20 cm (mean, 5.8 cm). For the 19 malignant PT, the patient age range was 30 to 76 years (mean, 49.8
y), and the tumor size range was 2 to 22 cm (mean, 6.8 cm). The mean age of patients increased with the degree of malignancy of PT. The difference between age of patients of the benign and
malignant groups was significant (_P_ = .0008), but no statistical significance was demonstrated between the benign and borderline groups and between the borderline and malignant groups. The
sizes of the PTs also increased with increasing degree of malignancy, but the differences were not statistically significant. For the p53 protein staining of all cases, 60 cases (42%)
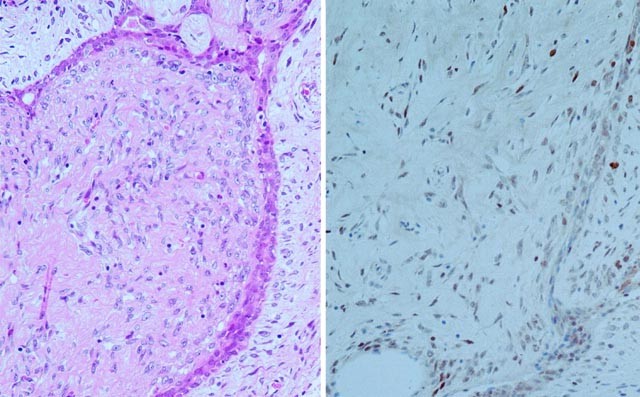
scored 0; 55 cases (38%) scored 1; 18 cases (13%) scored 2 (Figs. 1 and 2); and 10 cases (7%) scored 3 (Fig. 3 and 4). In the 60 cases with score 0, 41 (68%) were benign, 16 (27%) were
borderline, and 3 (5%) were malignant. In the 55 cases with score 1, 37 (67%) were benign, 14 (25%) were borderline, and 4 (8%) were malignant. For the 18 cases with score 2, 9 (50%) were
benign, 6 (33%) were borderline, and 3 (17%) were malignant. For the 10 cases with score 3, 1 (10%) was borderline and 9 (90%) were malignant. For the 87 benign PTs, 41 cases (47%) scored 0,
37 cases (43%) scored 1, and 9 cases (10%) scored 2. The mean score for benign PTs was 0.63. For the 37 borderline PTs, 16 cases (43%) scored 0, 14 cases (38%) scored 1, 6 cases (16%)
scored 2, and 1 case (3%) scored 3. The mean score for borderline PTs was 0.78. For the 19 malignant PTs, 3 cases (16%) scored 0, 4 cases (21%) scored 1, 3 cases (16%) scored 2, and 9 cases
(47%) scored 3. The mean score for malignant PTs was 1.95. Using ANOVA, the scores were significantly different among the three groups (_P_ < .0001) when considering the diagnosis with
p53 score. We further analyzed whether the p53 score was related to any of the histologic parameters or whether the p53 score was independent of these parameters. A logistic regression model
was used, taking into consideration the presence of the five histologic variables that were used for establishment of diagnosis. The correlation of p53 score with diagnosis now became
statistically not significant (_P_ = .187). Of the five histologic parameters analyzed, four of them (cellularity, nuclear pleomorphism, stromal overgrowth, and margin pattern) were not
associated with p53 score; only mitotic count showed a relationship with p53 score (_P_ = .0309). In fact, if we remove the mitotic count in the first logistic regression model, the
association between p53 and diagnosis became significant (_P_ = .0055), indicating that the information given by p53 and mitotic count were overlapping. Of the 137 patients, 17 were lost to
follow-up. For the remaining 120 patients, the follow-up period starting from the occurrence of the initial PT ranged from 2 to 300 months, with a mean of 68 months. One hundred patients
were well and did not have any recurrence, 18 had recurrences, and 2 had distant metastases. For the 18 patients with recurrences, 14 had one recurrence, and 4 had two recurrences. If only
the first recurrences were considered, the recurrence interval after the initial tumor was 6 to 204 months, with a mean of 36 months. One case had a recurrence interval of 204 months, and
for all others, the recurrence occurred within 72 months. For the second recurrence, the range was 24 to 240 months (mean, 91.8 mo). All the second recurrences occurred within 12 to 75
months after the first recurrence. For the disease outcome, one of the two patients with distant metastases died 2 years after diagnosis. For these two patients, one did not have histologic
assessment as the metastases were diagnosed radiologically. For the other case, the metastasis was composed of epithelioid malignant stromal cells with brisk mitoses. All other patients were
alive at the end of the follow-up period. Among the recurrences, six recurrent tumors (five first recurrences and one second recurrence from five patients) together with the initial PT were
available for review. The initial tumor was benign in one, borderline in two, and malignant in two cases. The benign PTs recurred as benign, but the recurrences from the borderline and
malignant groups recurred as all categories. No definite relationship could be established between the grade of the initial and the recurrent tumors. Analysis of the histologic variables and
p53 scores using logistic regression showed that most of these are not predictive of the occurrence of recurrence. Only the mitotic count was related to recurrence (_P_ = .0062). When we
compared the histologic parameters, tumor sizes, and p53 protein expressions of the recurrences and the nonrecurrent PTs using the _t_ test, the recurrences had higher degree of nuclear
pleomorphism, cellularity, stromal overgrowth and mitoses, but these were not statistically significant (_P_ = .1076– .2305). DISCUSSION The division of PTs into benign, borderline, and
malignant is essentially arbitrary because these lie along a histologic continuum rather than discreet histologic categories. Classification into benign and malignant PTs uses a combination
of histologic criteria, and for cases that fulfill some but not all malignant criteria, they are labeled as of borderline malignancy. This continuum is well illustrated by the fact that in
different large series, the proportion of borderline PTs differed significantly, ranging from 11 to 42%, with the malignant PTs ranging from 5 to 45% (13, 14, 15, 16, 17, 18). The findings
in the current series fell within these ranges. This division is nevertheless important because malignant PT has higher potential to metastasize. Additional factors that have been studied to
assist in the differentiation of different categories of PT included p53, CD34, bcl-2, Ki-67, endothelin 1, Factor XIIIa, and microvessel density (19, 20, 21, 22, 23). Studies evaluating
p53 protein expression in PT are few in the literature. Most of the series included limited number of cases (<20) of PT (10, 12, 14), whereas two studies were more comprehensive and
included 57 (11) and 118 (13) cases, respectively. Some of the smaller series also included only benign and malignant but not borderline PT in the analysis. The results of p53 protein
expression from these studies were variable. In some studies, p53 protein expression was exclusively present in malignant PT but not in benign PT or fibroadenomas (10, 12), and some authors
further distinguished the immunostaining patterns of weak and diffuse strong positivity (12). In other series with more cases and including borderline PT, the findings were less clear-cut,
showing p53 protein expression in malignant, borderline (11), and even benign (13, 14) PT. In all these studies, the percentage of PTs with p53 protein expression differed between different
grades of malignancy, ranging from 14 to 86% in malignant PTs, to 18 to 25% in borderline PTs, and to 0 to 10% in benign PTs (11, 14). In all individual studies, the percentage of p53
protein expression increased with ascending degree of malignancy. Our results demonstrated several findings. First, we showed that with increasing degree of malignancy, a concurrent increase
in p53 protein expression was detected, both in terms of the intensity and the percentage of cells. This observation is in agreement with the findings in other series (10, 11, 12, 13, 14).
We also demonstrated that p53 protein expression correlated strongly with the mitotic count but not with the diagnosis in the presence of mitotic count. This indicated that p53 expression
overlapped with mitoses and was not an independent factor in predicting malignancy in PTs. This is expected as the mechanism of p53 overexpression is related to controlled apoptosis in
response to DNA damage. As was previously demonstrated in a smaller series (11), our results showed that p53 protein expression was statistically significantly related to the mitotic
activity of the phyllodes tumor but did not predict outcome in terms of recurrence or mortality. Although other authors have reported an association of p53 expression with most histologic
features (stromal overgrowth, nuclear pleomorphism, infiltrative margin), this was not observed in our study. Immunostaining for p53 protein expression can be of utility in the diagnosis of
the malignant cases. If only diffuse strong positivity for p53 protein expression is considered, almost half of the malignant PTs exhibited this high level of expression, whereas only 3% of
the borderline and none of the benign PTs showed such strong expression. This finding echoes those of other authors (10, 11, 12, 13, 14), who demonstrated strong positive staining for
malignant PTs. For the larger series that included borderline cases (11, 13, 14), the percentage of positivity of borderline tumors ranged from 0 to 25%, compared with a single case of a
total of 37 cases (3%) in the current series. The specificity of diffuse strong p53 protein staining in malignant PTs in our series is 99%; sensitivity, 47%; positive predictive value, 90%;
and negative predictive value, 92.5%. This indicates that strong diffuse p53 staining is useful and specific in diagnosing malignant PTs with very high positive and negative predictive
values. In predicting recurrences, we showed that most histologic criteria and p53 protein expression were not useful, with only mitotic count correlating with recurrences. In comparing the
recurrences and the nonrecurrent tumors, the recurrent tumors showed higher degree of stromal cellularity, pleomorphism, overgrowth, and mitoses, but these were not statistically
significant. We believe that if more cases of recurrent tumors were available, statistical significance may be established for these parameters, which are at best weak predictors for
recurrence. p53 protein expression is not useful in predicting outcome. The underlying molecular mechanism of causation and progression of PTs remains poorly understood. In the literature, a
few cytogenetic studies by comparative genomic hybridization (CGH) showed 3p loss and 1q gain to be the more common abnormalities in PTs (24, 25); however, these changes were not
consistent. The role of p53 mutation in the malignant transformation of PTs has yet to be elucidated, although point mutation has been found to be the responsible mechanism in a case of a
benign PT that transformed into a malignant PT (26). It would appear that with the strong and diffuse staining for p53 protein expression present in mostly malignant but not benign or
borderline PTs, such mutations occur as late events in tumor progression. Furthermore, the presence of p53 protein expression in only some of the malignant PTs in all series suggests that
changes in other tumor suppressor genes may also play a significant role in the malignant transformation. This may also partly explain the lack of power of p53 protein expression in
predicting recurrences and outcome. Although the use of diffuse strong staining for p53 protein can be used as a “soft sign” in the diagnosis of malignant PT, further molecular studies are
warranted to elucidate the mechanism, including that of p53 mutations, involved in the pathogenesis and progression of PT. REFERENCES * Cole-Beuglet C, Soriano R, Kurtz AB, Meyer JE, Kopans
DB, Goldberg BB . Ultrasound, X-ray mammography, and histopathology of cystosarcoma phyllodes. _Radiology_ 1983; 146: 481–486. Article CAS Google Scholar * Azzopardi JG . Sarcoma of the
breast.In: Bennington J, editor. _Problems in breast pathology. Vol II. Major problems in Pathology_. Philadelphia: Saunders; 1979.p. 355–359. Google Scholar * Palmer ML, De Risi DC,
Pelikan A, Patel J, Nemoto T, Rosner D, _et al_. Treatment options and recurrence potential for cystosarcoma phyllodes. _Surg Gynecol Obstet_ 1990; 170: 193–196. CAS PubMed Google Scholar
* Kario K, Maeda S, Mizuno Y, Makino Y, Tankawa H, Kitazawa S . Phyllodes tumor of the breast: a clinicopathologic study of 34 cases. _J Surg Oncol_ 1990; 45: 46–51. Article CAS Google
Scholar * Rowell MD, Perry RR, Hsiu JG, Barranco SC . Phyllodes tumors. _Am J Surg_ 1993; 165: 376–379. Article CAS Google Scholar * Rosen PP . Breast pathology. 2nd ed. Philadelphia:
Lippincott-Raven; 2001.p. 176–197. * Lane DP . p53 and human cancers. _Br Med Bull_ 1994; 50: 582–599. Article CAS Google Scholar * Carder P, Wyllie AH, Purdie CA, Morris RG, White S,
Piris J, _et al_. Stabilised p53 facilitates aneuploid clonal divergence in colorectal cancer. _Oncogene_ 1993; 8: 1397–1401. CAS PubMed Google Scholar * Neshat K, Sanchez CA, Galipeau
PC, Blount PL, Levine DS, Joslyn G, _et al_. P53 mutations in Barrett’s adenocarcinoma and high-grade dysplasia. _Gastroenterology_ 1994; 106: 1589–1595. Article CAS Google Scholar *
Millar EK, Beretov P, Marr P, Sarris M, Clarke RA, Kearsley JH, _et al_. Malignant phyllodes tumours of the breast display increased stromal p53 protein expression. _Histopathology_ 1999;
34: 491–496. Article CAS Google Scholar * Feakins RM, Mulcahy HE, Nickols CD, Wells CA . p53 expression in phyllodes tumours is associated with histological features of malignancy but
does not predict outcome. _Histopathology_ 1999; 35: 162–169. Article CAS Google Scholar * Kim CJ, Kim WH . Patterns of p53 expression in phyllodes tumors of the breast—an
immunohistochemical study. _J Korean Med Sci_ 1993; 8: 325–328. Article CAS Google Scholar * Niezabitowski A, Lackowska B, Rys J, Kruczak A, Kowalska T, Mitus J, _et al_. Prognostic
evaluation of proliferative activity and DNA content in the phyllodes tumor of the breast: immunohistochemical and flow cytometric study of 118 cases. _Breast Cancer Res Treat_ 2001; 65:
77–85. Article CAS Google Scholar * Kuenen-Boumeester V, Henzen-Logmans SC, Timmermans MM, van Staveren IL, Van Geel A, Peeterse HJ, _et al_. Altered expressiopn of p53 and its regulated
proteins in phyllodes tumours of the breast. _J Pathol_ 1999; 189: 169–175. Article CAS Google Scholar * Shabalova IP, Chemeris GJ, Ermilova VD, Rodionova LM, Pavlikova NA, Syrjanen KJ .
Phyllodes tumour: cytologic and histologic presentation of 22 cases, and immunohistochemical demonstration of p53. _Cytopathology_ 1997; 8: 177–187. Article CAS Google Scholar * Suo Z,
Nesland JM . Phyllodes tumor of the breast: EGFR family expression and relation to clinicopathological features. _Ultrastruct Pathol_ 2000; 24: 371–381. Article CAS Google Scholar *
Reinfuss M, Mitus J, Duda K, Stelmach A, Rys J, Smolak K . The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 179 cases. _Cancer_ 1996; 77: 910–916.
Article CAS Google Scholar * Cohn-Cedermark G, Rutqvist LE, Rosendahl I, Silfversward C . Prognostic factors in cystosarcoma phyllodes. A clinicopathologic study of 77 patients. _Cancer_
1991; 68: 2017–2022. Article CAS Google Scholar * Moore T, Lee AHS . Expression of CD34 and bcl-2 in phyllodes tumours, fibroadenomas and spindle cell lesions of the breast.
_Histopathology_ 2001; 38: 62–67. Article CAS Google Scholar * Silverman JS, Tamsen A . Mammary fibroadenoma and some phyllodes tumour stroma are composed of CD34+ fibroblasts and factor
XIIIa dendrophages. _Histopathology_ 1996; 29: 411–419. Article CAS Google Scholar * Yamashita J, Ogawa M, Egami H, Matsuo S, Kiyohara H, Inada K, _et al_. Abundant expression of
immunoreactive endothelin 1 in mammary phyllodes tumor: possible paracrine role of endothelin 1 in the growth of stromal cells in phyllodes tumor. _Cancer Res_ 1992; 52: 4046–4049. CAS
PubMed Google Scholar * Gatalica Z, Lucio E, Finkelstein S, Palazzo J, Tawlik O . The role of p53 mutation and Ki-67 proliferation index in the diagnosis and progression of phyllodes tumor
of the breast [abstract]. _Lab Invest_ 1999; 79: 21A. Google Scholar * Tse GM, Ma TK, Chan KF, Law BK, Chen MH, Li KH, _et al_. Increased microvessel density in malignant and borderline
mammary phyllodes tumours. _Histopathology_ 2001; 38: 67–70. Article Google Scholar * Sawyer EJ, Hanby AM, Ellis P, Lakhani SR, Ellis IO, Boyle S, _et al_. Molecular analysis of phyllodes
tumors reveals distinct changes in the epithelial and stromal components. _Am J Pathol_ 2000; 156: 1093–1098. Article CAS Google Scholar * Woolley PV, Gollin SM, Riskalla W, Finkelstein
S, Stefanik D, Riskalla L, _et al_. Cytogenetics, immunostaining for fibroblastic growth factors, p53 sequencing and clinical features of two cases of cystosarcoma phyllodes. _Mol Diagn_
2000; 5: 179–190. Article CAS Google Scholar * Gatalica Z, Finkelstein S, Lucio E, Tawfik O, Palazzo J, Hightower B, _et al_. p53 protein expression and gene mutation in phyllodes tumors
of the breast. _Pathol Res Pract_ 2001; 197: 183–187. Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Anatomical and Cellular
Pathology, Chinese University of Hong Kong, Hong Kong Gary M K Tse FRCPC & Fred Y L Kung B.Sc. * Department of Surgery, Chinese University of Hong Kong, Hong Kong Bonita K B Law FRCS *
Prince of Wales Hospital, and Statistics, Chinese University of Hong Kong, Hong Kong Tai-shing Lau Ph.D. * Department of Pathology, National University Hospital, Singapore Thomas C Putti
Dip. Am. Bd. (Path.) * Department of Pathology, and Department of Anatomical Pathology, University of Sydney, Royal Prince Alfred Hospital, Sydney, Australia Richard A Scolyer FRCPA & C
Soon Lee FRCPA Authors * Gary M K Tse FRCPC View author publications You can also search for this author inPubMed Google Scholar * Thomas C Putti Dip. Am. Bd. (Path.) View author
publications You can also search for this author inPubMed Google Scholar * Fred Y L Kung B.Sc. View author publications You can also search for this author inPubMed Google Scholar * Richard
A Scolyer FRCPA View author publications You can also search for this author inPubMed Google Scholar * Bonita K B Law FRCS View author publications You can also search for this author
inPubMed Google Scholar * Tai-shing Lau Ph.D. View author publications You can also search for this author inPubMed Google Scholar * C Soon Lee FRCPA View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Gary M K Tse FRCPC. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Tse, G., Putti, T., Kung, F. _et al._ Increased p53 Protein Expression in Malignant Mammary Phyllodes Tumors. _Mod Pathol_ 15, 734–740 (2002).
https://doi.org/10.1097/01.MP.0000018978.75312.5C Download citation * Accepted: 03 April 2002 * Published: 01 July 2002 * Issue Date: July 2002 * DOI:
https://doi.org/10.1097/01.MP.0000018978.75312.5C SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Breast * Immunohistochemistry * Malignant * p53 *
Pathology * Phyllodes tumor
