Androgen decreases osteoprotegerin expression in prostate cancer cells
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

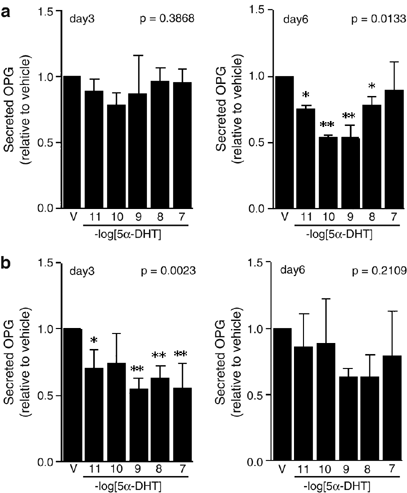
ABSTRACT Osteoprotegerin (OPG), a key regulator of bone resorption, is hypothesized to have a role in prostate cancer (CaP) bone metastasis. As advanced CaP is treated by androgen ablation,
we examined if androgen modulates OPG expression by CaP cell lines _in vitro_. Basal levels of secreted OPG protein were significantly greater in androgen-independent PC-3 cells compared
with androgen-responsive LNCaP-FGC cells (_P_<0.001); OPG was not detected in the androgen-responsive CaP cell lines LAPC-4 or DuCaP. Treatment with 5_α_-dihydrotestosterone (5_α_-DHT)
significantly decreased OPG protein levels in both PC-3 and LNCaP-FGC, with maximal suppression using 10−9–10−7 M 5_α_-DHT in PC-3 (_P_<0.01; day 3), and using 10−10–10−9 M 5_α_-DHT in
LNCaP-FGC cells (_P_<0.01; day 6). OPG messenger RNA levels were not significantly altered by this 5_α_-DHT treatment. Co-treatment with 10−6 M flutamide blocked 5_α_-DHT inhibition of
OPG protein expression in LNCaP-FGC cells. These data suggest that androgen may modulate OPG protein levels in CaP cells lines _in vitro_ using a post-transcriptional mechanism. Access
through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal
Receive 4 print issues and online access $259.00 per year only $64.75 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may
be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
SIMILAR CONTENT BEING VIEWED BY OTHERS STATINS REDUCE CASTRATION-INDUCED BONE MARROW ADIPOSITY AND PROSTATE CANCER PROGRESSION IN BONE Article 14 June 2021 Α-LIPOIC ACID MODULATES PROSTATE
CANCER CELL GROWTH AND BONE CELL DIFFERENTIATION Article Open access 22 February 2024 GDF15 PROMOTES PROSTATE CANCER BONE METASTASIS AND COLONIZATION THROUGH OSTEOBLASTIC CCL2 AND RANKL
ACTIVATION Article Open access 20 January 2022 REFERENCES * Carlin BI, Andriole GL . The natural history, skeletal complications, and management of bone metastases in patients with prostate
carcinoma. _Cancer_ 2000; 88: 2989–2994. Article CAS Google Scholar * McMurtry CT, McMurtry JM . Metastatic prostate cancer: complications and treatment. _J Am Geriatr Soc_ 2003; 51:
1136–1142. Article Google Scholar * Morony S, Capparelli C, Sarosi I, Lacey DL, Dunstan CR, Kostenuik PJ . Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in
syngeneic and nude mouse models of experimental bone metastasis. _Cancer Res_ 2001; 61: 4432–4436. CAS Google Scholar * Penno H, Silfverswärd C-J, Frosta A, Brändström H, Nilsson O,
Ljunggren Ö . Osteoprotegerin secretion from prostate cancer is stimulated by cytokines, _in vitro_. _Biochem Biophys Res Commun_ 2002; 293: 451–455. Article CAS Google Scholar * Pollen
JJ, Reznek RH, Talner LB . Lysis of osteoblastic lesions in prostatic cancer: a sign of progression. _AJR Am J Roentgenol_ 1984; 142: 1175–1179. Article CAS Google Scholar * Zhang J, Dai
J, Qi Y, Lin DL, Smith P, Strayhorn C _et al_. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. _J Clin Invest_ 2001; 107:
1235–1244. Article CAS Google Scholar * Balk SP . Androgen receptor as a target in androgen-independent prostate cancer. _Urology_ 2002; 60: 132–138. Article Google Scholar * Hara T,
Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M _et al_. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. _Cancer Res_ 2003; 63: 149–153.
CAS Google Scholar * Orwoll ES, Klein RF . Osteoporosis in men. _Endocr Rev_ 1995; 16: 87–116. Article CAS Google Scholar * Syed F, Khosla S . Mechanisms of sex steroid effects on bone.
_Biochem Biophys Res Commun_ 2005; 328: 688–696. Article CAS Google Scholar * Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R _et al_. Osteoprotegerin: a novel secreted
protein involved in the regulation of bone density. _Cell_ 1997; 89: 309–319. Article CAS Google Scholar * Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano N, Fujise K _et al_. Identity
of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis _in vitro_. _Endocrinology_ 1998; 139: 1329–1337. Article
Google Scholar * Lacey D, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T _et al_. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. _Cell_
1998; 93: 165–176. Article CAS Google Scholar * Brown JM, Corey E, Lee ZD, True LD, Yun TJ, Tondravi M _et al_. Osteoprotegerin and RANK ligand expression in prostate cancer. _Urology_
2001; 57: 611–616. Article CAS Google Scholar * Brown JM, Vessella RL, Kostenuik PJ, Dunstan CR, Lange PH, Corey E . Serum osteoprotegerin levels are increased in patients with advanced
prostate cancer. _Clin Cancer Res_ 2001; 7: 2977–2983. CAS Google Scholar * Jung K, Lein M, von Hösslin K, Brux B, Schnorr D, Loening SA _et al_. Osteoprotegerin in serum as a novel marker
of bone metastatic spread in prostate cancer. _Clin Chem_ 2001; 47: 2061–2063. CAS Google Scholar * Jung K, Stephan C, Semjonow A, Lein M, Schnorr D, Loening SA . Serum osteoprotegerin
and receptor activator of nuclear factor-_κ_B ligand as indicators of disturbed osteoclastogenesis in patients with prostate cancer. _J Urol_ 2003; 170: 2302–2305. Article Google Scholar *
Eaton CL, Wells JM, Holen I, Croucher PI, Hamdy FC . Serum osteoprotegerin (OPG) levels are associated with disease progression and response to androgen ablation in patients with prostate
cancer. _Prostate_ 2004; 59: 304–310. Article CAS Google Scholar * Holen I, Croucher PI, Hamdy FC, Eaton CL . Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells.
_Cancer Res_ 2002; 62: 1619–1623. CAS Google Scholar * Hofbauer LC, Hicok KC, Chen D, Khosla S . Regulation of osteoprotegerin production by androgens and anti-androgens in human
osteoblastic lineage cells. _Eur J Endocrinol_ 2002; 147: 269–273. Article CAS Google Scholar * Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS . Phenol red in tissue culture media
is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. _Proc Natl Acad Sci USA_ 1986; 83: 2496–2500. Article CAS Google Scholar * Buchanan G, Craft
PS, Yang M, Cheong A, Prescott J, Jia L _et al_. PC-3 cells with enhanced androgen receptor signaling: a model for clonal selection in prostate cancer. _Prostate_ 2004; 60: 352–366. Article
CAS Google Scholar * Marreiros A, Czolij R, Yardley G, Crossley M, Jackson P . Identification of regulatory regions within the KAI1 promoter: a role for binding of AP1, AP2 and p53.
_Gene_ 2003; 302: 155–164. Article CAS Google Scholar * Tilley WD, Bentel JM, Aspinall JO, Hall RE, Horsfall DJ . Evidence for a novel mechanism of androgen resistance in the human
prostate cancer cell line, PC-3. _Steroids_ 1995; 60: 180–186. Article CAS Google Scholar * Lin MF, Meng TC, Rao PC, Chang C, Schönthal AH, Lin FF . Expression of human prostatic acid
phosphatase correlates with androgen-stimulated cell proliferation in prostate cancer cell lines. _J Biol Chem_ 1998; 273: 5939–5947. Article CAS Google Scholar * Alimirah F, Chen J,
Basrawala Z, Xin H, Choubey D . DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: implications for the androgen receptor functions and regulation. _FEBS Lett_ 2006;
580: 2294–2300. Article CAS Google Scholar * Yuan S, Trachtenberg J, Mills GB, Brown TJ, Xu F, Keating A . Androgen-induced inhibition of cell proliferation in an androgen-insensitive
prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. _Cancer Res_ 1993; 53: 1304–1311. CAS Google Scholar * Garcia-Arenas R, Lin F-F, Lin D, Jin
LP, Shih CC, Chang C _et al_. The expression of prostatic acid phosphatase is transcriptionally regulated in human prostate carcinoma cells. _Mol Cell Endocrinol_ 1995; 111: 29–37. Article
CAS Google Scholar * Benten WP, Lieberherr M, Sekeris CE, Wunderlich F . Testosterone induces Ca2+ influx via non-genomic surface receptors in activated T cells. _FEBS Lett_ 1997; 407:
211–214. Article CAS Google Scholar * Wehling M . Specific, nongenomic actions of steroid hormones. _Annu Rev Physiol_ 1997; 59: 365–393. Article CAS Google Scholar * Nadal A, Rovira
JM, Laribi O, Leon-quinto T, Andreu E, Ripoll C _et al_. Rapid insulinotropic effect of 17_β_-estradiol via a plasma membrane receptor. _FASEB J_ 1998; 12: 1341–1348. Article CAS Google
Scholar * Benten WP, Lieberherr M, Stamm O, Wrehlke C, Guo Z, Wunderlich F . Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. _Mol Biol
Cell_ 1999; 10: 3113–3123. Article CAS Google Scholar * Lyng FM, Jones GR, Rommerts FF . Rapid androgen actions on calcium signaling in rat sertoli cells and two human prostatic cell
lines: similar biphasic responses between 1 picomolar and 100 nanomolar concentrations. _Biol Reprod_ 2000; 63: 736–747. Article CAS Google Scholar * Papakonstanti EA, Kampa M, Castanas
E, Stournaras C . A rapid, nongenomic, signaling pathway regulates the actin reorganization induced by activation of membrane testosterone receptors. _Mol Endocrinol_ 2003; 17: 870–881.
Article CAS Google Scholar * Steinsapir J, Socci R, Reinach P . Effects of androgen on intracellular calcium of LNCaP cells. _Biochem Biophys Res Commun_ 1991; 179: 90–96. Article CAS
Google Scholar * Kampa M, Papakonstanti EA, Alexaki VI, Hatzoglou A, Stournaras C, Castanas E . The opioid agonist ethylketocyclazocine reverts the rapid, non-genomic effects of membrane
testosterone receptors in the human prostate LNCaP cell line. _Exp Cell Res_ 2004; 294: 434–445. Article CAS Google Scholar * Bellido T, Jilka RL, Boyce BF, Girasole G, Broxmeyer H,
Dalrymple S _et al_. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. _J Clin Invest_ 1995; 95: 2886–2895. Article CAS Google
Scholar * Hofbauer LC, Khosla S . Androgen effects on bone metabolism: recent progress and controversies. _Eur J Endocrinol_ 1999; 140: 271–286. Article CAS Google Scholar * Pederson L,
Kremer M, Judd J, Pascoe D, Spelsberg TC, Riggs BL _et al_. Androgens regulate bone resorption activity of isolated osteoclasts _in vitro_. _Proc Natl Acad Sci USA_ 1999; 96: 505–510.
Article CAS Google Scholar * Gennari L, Nuti R, Bilezikian JP . Aromatase activity and bone homeostasis in men. _J Clin Endocrinol Metab_ 2004; 89: 5898–5907. Article CAS Google Scholar
* Wittrant Y, Théoleyre S, Chipoy C, Padines M, Blanchard F, Heymann D _et al_. RANKL/RANK/OPG: new therapeutic targets in bone tumours and associated osteolysis. _Biochim Biophys Acta_
2004; 1704: 49–57. CAS Google Scholar * Blair JM, Zhou H, Seibel MJ, Dunstan CR . Mechanisms of disease: roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. _Nat
Clin Pract Oncol_ 2006; 3: 41–49. Article CAS Google Scholar * Grimaud E, Soubigou L, Couillaud S, Coipeau P, Moreau A, Passuti N _et al_. Receptor activator of nuclear factor _κ_B ligand
(RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. _Am J Pathol_ 2003; 163: 2021–2031. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Dr
Barbara Szymanska and Elizabeth Kingsley for their excellent technical assistance. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Oncology Research Centre, Prince of Wales Hospital, Randwick,
New South Wales, Australia K Vandyke, P Jackson, A Rowe, P J Russell & J M Blair * Faculty of Medicine, University of New South Wales, Kensington, New South Wales, Australia K Vandyke,
P Jackson, A Rowe, P J Russell & J M Blair * School of Biotechnology and Biomolecular Sciences, University of New South Wales, Kensington, New South Wales, Australia K Vandyke Authors *
K Vandyke View author publications You can also search for this author inPubMed Google Scholar * P Jackson View author publications You can also search for this author inPubMed Google
Scholar * A Rowe View author publications You can also search for this author inPubMed Google Scholar * P J Russell View author publications You can also search for this author inPubMed
Google Scholar * J M Blair View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to J M Blair. RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Vandyke, K., Jackson, P., Rowe, A. _et al._ Androgen decreases osteoprotegerin expression in prostate cancer cells. _Prostate
Cancer Prostatic Dis_ 10, 160–166 (2007). https://doi.org/10.1038/sj.pcan.4500927 Download citation * Received: 18 September 2006 * Accepted: 13 October 2006 * Published: 26 December 2006 *
Issue Date: 01 May 2007 * DOI: https://doi.org/10.1038/sj.pcan.4500927 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * osteoprotegerin *
androgen * bone metastasis * bone resorption
