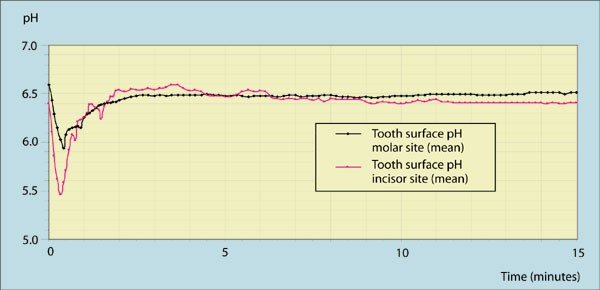
Tooth surface ph during drinking of black tea
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT OBJECTIVES To investigate the composition of black tea in terms of its erosive potential. To determine the pH profile at the palatal surface of anterior and posterior sites of the
dentition after drinking black tea. METHODS Tea solution was analysed for its pH and anion composition to provide information on its acid content. A group of ten healthy subjects, aged 21–23
years were monitored for tooth surface pH on the palatal aspects of the maxillary anterior dentition and the maxillary molar dentition after drinking tea using a micro-pH electrode mounted
on a vinyl splint. RESULTS The pH of the tea solution was 4.9 and the major anions detected were oxalate and citrate. Tooth surface pH monitoring indicated that only small decreases in pH of
less than 1pH unit were observed after drinking tea and the minimum mean pH reached was 5.45. Maximum decrease in pH was observed after 20–25 seconds and resting pH levels were restored
within approximately 2 minutes after drinking. CONCLUSION The pH and anion profile of black tea are indicative of low acid composition. The very small pH decreases observed at the tooth
surface after drinking tea indicate that it may be safely recommended as a substitute for more acidic drinks as a part of preventive measures for dental erosion. You have full access to this
article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS PLANT EXTRACTS HAVE DUAL MECHANISM ON THE PROTECTION AGAINST DENTINE EROSION: ACTION ON THE DENTINE
SUBSTRATE AND MODIFICATION OF THE SALIVARY PELLICLE Article Open access 01 May 2023 EFFECTS OF A MOUTHWASH CONTAINING _LESPEDEZA CUNEATA_ EXTRACT ON RISK OF DENTAL CARIES: A RANDOMIZED,
PLACEBO-CONTROLLED CLINICAL TRIAL Article Open access 01 December 2022 THE BLOOD PRESSURE LOWERING EFFECT OF BEETROOT JUICE IS IMPAIRED IN PERIODONTITIS AND RECOVERED AFTER PERIODONTAL
TREATMENT Article Open access 09 January 2025 MAIN A number of studies have reported significant levels of dental erosion amongst both children and adults in the UK.1,2,3,4,5,6 Dental
erosion may be of both intrinsic7 and extrinsic,8 ie endogenous (recurrent vomiting, regurgitation, reflux) or exogenous (principally diet), origin and the relative importance of these two
origins will be determined by a number of factors. An extrinsic origin for erosion will be influenced both by dietary and host factors, the former of which provides a possible way to reduce
the prevalence and severity of the condition. A variety of dietary components may contribute to erosion,4,6,9,10,11,12,13 but acidic drinks appear to be of particular importance. An
association between consumption of acidic drinks and erosion has been demonstrated4,6,14,15and attempts have been made to re-formulate citrus-based drinks to reduce their erosive
potential.16 However, the increasing consumption of these acidic drinks17 continues to be a matter of concern in terms of erosion. There is a need to identify drinks of low erosive
potential, which may be safely recommended to patients for substitution for more acidic drinks in the diet. Tea has long been an important fluid component of the diet in the UK. Whilst
traditionally it has largely been consumed as a hot beverage, the American practice of consuming iced tea without milk is also appearing in the UK. The majority of the tea consumed in the UK
is black tea rather than the green variant, which is more popular in the Far East. Much of the tea habitually consumed in the UK is with the addition of milk, which may confer nutritional
benefits on the beverage. Tea has a complex composition and its consumption has been recognised as having some beneficial dental effects because of its appreciable fluoride content.18,19
However, little is known of the acid content of black tea and its influence on oral acidity during consumption. The aim of the present study was to characterise the acid composition of black
tea and to assess the influence of its consumption on tooth surface pH in human subjects to provide a basis for dietary advice to patients. MATERIALS AND METHODS Tea infusion solutions (1%
w/v) were prepared from black tea (World Blend – Tetley USA Inc) using a commercial Bunn-O-Matic tea brewer (Bunn-O-Matic Corp, USA) with 40 g tea leaves and 4 L double distilled deionised
water. Solutions were prepared fresh daily and allowed to cool to room temperature prior to use. The anion composition of the tea solution was quantitated by ion chromatography using a
Dionex DX-500 ion chromatograph and an AS15 high capacity separation column eluted with a water– potassium hydroxide gradient to assess possible acid components (Dionex UK Ltd, Camberley,
Surrey, GU15 2XA, UK). Separated components were detected by conductivity with ion suppression and the digitised data quantitated by calibration of the system with standard solutions of the
acids. Ten healthy subjects, aged 21–23 years, with good standards of oral hygiene were monitored for tooth surface pH after drinking tea. Ethical approval for the study was received from
the South Birmingham Local Research Ethics Committee. The influence of black tea on tooth surface pH was assessed using an _in situ_ pH monitoring approach previously reported by our
group.20 Essentially, this involved the manufacture of vinyl splints from individual alginate impressions and plaster casts of the subjects' maxillary dentition. Short lengths of
small-bore plastic tubing were mounted on the splints at two positions with cold cure acrylic to provide mounting points for a micro-pH electrode (0.6 mm diameter). The micro-electrode was
of the type routinely used for oesophageal pH monitoring. The two positions were those previously chosen to reflect areas of higher and lower dietary erosion – palatal aspect of the
maxillary anterior dentition and palatal aspect of the maxillary molar region. Using a skin reference electrode and patient-isolated pH meter, the pH at the dentition surface was monitored
in subjects under resting conditions and after drinking tea. The resting pH essentially provided an internal control of tooth surface pH in the absence of ingested dietary acids. The pH
changes in the presence of such acids have been previously reported.20 All measurements were made at the same time of day (midday) to avoid diurnal variation and subjects refrained from
drinking or eating for 3 hours prior to the experiment. Each subject was calibrated to the pH meter with two standard pH buffer solutions and after assessment of resting pH, the subject
drank 100 ml of the tea solution and the pH was recorded for periods of up to 20 minutes. RESULTS The pH of the tea solution was 4.9. The anion profile for the tea solution indicated that
oxalate and citrate were the major anions detected (Table 1). Monitoring of tooth surface pH indicated that only small decreases in pH were detected at both palatal incisor and molar sites
after drinking tea (Fig. 1). The mean resting pH for the subjects at the two sites was: incisor = 6.40, molar = 6.59. The maximum decrease in pH was observed at 20 and 25 seconds
respectively for the two sites, which represented: incisor = 0.94, molar = 0.65. These corresponded to minimum pHs of 5.45 and 5.95 respectively at these two sites. The inter-subject
variation in pH both at rest and after drinking tea was small. The standard deviation for the pH at both sites in the group were generally in the range 0.30 to 0.35 and did not exceed 0.65
at any time point. The minimum and maximum pH decreases at the incisor site were 0.82 and 1.60 and at the molar site were 0.45 and 0.98 respectively for the subject group. Resting pH was
restored within approximately 2 minutes at both sites after drinking the tea. The mean time for the drinking of the tea was 27.3 and 22.0 seconds respectively for the incisor and molar site
experiments. DISCUSSION The increasing awareness of dental erosion and the need for good preventive advice21 requires that practitioners and other healthcare professionals should be aware of
what drinks can safely be recommended to patients. Such advice must be realistic and any recommendations should generally be as a part of a well-balanced diet. The present study has
demonstrated that black tea does not have a low pH in comparison with many of the beverages currently consumed and has an anion profile indicative of low acid composition. Citrate and
oxalate were the major anions present. Under acidic conditions, the concentrations of these anions would suggest molar concentrations of no greater than 0.23 and 1.3 mM for citric and oxalic
acids respectively. To put these figures in dietary context, tea would have less than 1% of the citric acid concentration found in pure orange juice. The low acidity of the tea is reflected
in the pH profiles at the dentition surface recorded. The decreases in pH observed after drinking tea were minimal compared with those previously observed after drinking 1% citric acid
(similar concentration to some fruit-based acidic drinks) where pHs as low as 2 were reached.20 Not only were the pH decreases very small but also, they occurred very rapidly (maximal at 20
to 25 seconds) and resting pH levels were restored within approximately 2 minutes. Although there was a greater decrease in the incisor region compared with the molar region, this would not
be clinically significant. Thus, drinking tea led to only small and short-lived decreases in pH at the tooth surface. It seems unlikely that such pH changes would be significant in the
development of erosive lesions and that tea could be safely recommended as a substitute for more acidic drinks. The limited sample of young, healthy subjects in the present study was
dictated by the nature of the pH monitoring procedures. However, there is little reason to expect that the results observed would differ from the general population unless salivary function
was compromised in some way. Diet can be influenced by life-style pressures and the American trend of consumption of iced tea and cold tea in soft-drink type cans may allow tea to be
successfully targeted at younger as well as older age groups. However, addition of lemon to tea to enhance flavouring should be avoided as should sugar, the latter of which may contribute to
dental caries. Further oral health benefits might also accrue from drinking tea as a result of its appreciable fluoride content.18,19 Despite these possible advantages of tea, excessive
consumption may lead to problems of staining of the dentition. Such staining is likely to be caused by interaction of components of the tea with both surface integuments like the acquired
salivary pellicle and possibly, the mineral crystals of dental enamel. Whilst there is no evidence to indicate that this staining is detrimental to tooth structure and function, it can
represent a cosmetic problem. In groups at risk of iron deficiency, eg young infants and the elderly, excessive consumption of tea should be avoided to prevent possible effects on intestinal
mineral absorption. However, such potential problems relate to excessive consumption and recommendation of any beverage should be as a part of a well balanced diet. Whilst erosion is
clearly multi-factorial in nature, dietary advice to reduce the consumption of acidic drinks may have significant benefits in its prevention. The present study has demonstrated that tea
could provide a useful substitute for more acidic drinks in the diet. REFERENCES * O'Brien M . Child Dental Health in the United Kingdom 1993. _Office of Population Censuses and
Surveys_. London: Her Majesty's Stationery Office, 1994. Google Scholar * Milosevic A, Young P J, Lennon M A . The prevalence of tooth wear in 14-year-old school children in Liverpool.
_Community Dent Health_ 1994; 11: 83–86. PubMed Google Scholar * Bartlett D W, Coward P Y, Nikkah C, Wilson R F . The prevalence of tooth wear in a cluster sample of adolescent
schoolchildren and its relationship with potential explanatory factors. _Br Dent J_ 1998; 184: 125–129. Article PubMed Google Scholar * Millward A, Shaw L, Smith A J, Rippin J W,
Harrington E . The distribution and severity of tooth wear and relationship between erosion and dietary constituents in a group of children. _Int J Paed Dent_ 1994; 4: 151–157. Article
Google Scholar * Al-Dlaigan Y H, Shaw L, Smith A J . Dental erosion in a group of British 14-year-old, school children. Part I : Prevalence and influence of differing socioeconomic
backgrounds. _Br Dent J_ 2000; in press. * Al-Dlaigan Y H, Shaw L, Smith A J . Dental erosion in a group of British 14-year-old, school children. Part II: Influence of dietary intake. _Br
Dent J_ 2000; in press. * Scheutzel P . Etiology of dental erosion – intrinsic factors. _Eur J Oral Sci_ 1996; 104: 178–190. Article PubMed Google Scholar * Zero D T . Etiology of dental
erosion – extrinsic factors. _Eur J Oral Sci_ 1996; 104: 162–177. Article PubMed Google Scholar * Levine R . S. Fruit juice erosion – an increasing danger. _J Dent_ 1973; 2: 85–88.
Article PubMed Google Scholar * Linkosalo E, Markkonam H . Dental erosion in relation to lactovegetarian diet. _Scand J Dent Res_ 1985; 93: 436–441. PubMed Google Scholar * Mueninghoff
L A, Johnson M H . Erosion: a case caused by unusual diet. _J Am Diet Assoc_ 1982; 104: 51–52. Article Google Scholar * Smith A J, Shaw L . Baby fruit juices and tooth erosion. _Br Dent J_
1987; 162: 65–67. Article PubMed Google Scholar * Harrison J L, Roder L B . Dental erosion caused by cola beverages. _Gen Dent_ 1991; 39: 23–24. PubMed Google Scholar * Johansson A K,
Johansson A, Birkhed D et al. Dental erosion associated with soft-drink consumption in young Saudi men. _Acta Odont Scand_ 1997; 55: 390–397. Article PubMed Google Scholar * Milosevic A,
Lennon M A, Fear S C . Risk factors associated with tooth wear in teenagers : a case control study. _Community Dent Health_ 1997; 14: 143–147. PubMed Google Scholar * West N X, Hughes J A,
Parker D M, Newcombe R G, Addy M . Development and evaluation of a low erosive blackcurrant drink. 2. Comparison with a conventional blackcurrant juice drink and orange juice. _J Dent_
1999; 27: 341–344. Article PubMed Google Scholar * British Soft Drinks Association. _Report of seminar in Heidelberg 1991_. Factsheet number. 1991: 9–7, 91. * Duckworth C S, Duckworth R .
The ingestion of fluoride in tea. _Br Dent J_ 1978; 145: 368–370. Article PubMed Google Scholar * Walters C B, Sherlock J C, Evans W H, Read I . Dietary intake of fluoride in the United
Kingdom and fluoride content of some foodstuffs. _J Sci Food Agricult_ 1983; 34: 523–528. Article Google Scholar * Millward A, Shaw L, Harrington E, Smith A J . Continuous monitoring of
salivary flow rate and pH at the surface of the dentition following consumption of acidic beverages. _Caries Res_ 1997; 31: 44–49. Article PubMed Google Scholar * Shaw L, Smith A J .
Dental erosion – the problem and some practical solutions. _Br Dent J_ 1999; 186: 115–118. PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors are grateful to the
International Steering Committee of the Tea Trade Health Research Association for their support of this study and to Dionex Ltd for their assistance with the ion chromatography. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Research Assistant, University of Birmingham, St Chads Queensway, Birmingham A Simpson * Senior Lecturer in Paediatric Dentistry, University of
Birmingham, St Chads Queensway, Birmingham L Shaw * Professor in Oral Biology, School of Dentistry, University of Birmingham, St Chads Queensway, Birmingham A J Smith Authors * A Simpson
View author publications You can also search for this author inPubMed Google Scholar * L Shaw View author publications You can also search for this author inPubMed Google Scholar * A J Smith
View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to A J Smith. ADDITIONAL INFORMATION REFEREED PAPER RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Simpson, A., Shaw, L. & Smith, A. Tooth surface pH during drinking of black tea. _Br Dent J_ 190, 374–376
(2001). https://doi.org/10.1038/sj.bdj.4800977 Download citation * Received: 31 July 2000 * Accepted: 12 October 2000 * Published: 14 April 2001 * Issue Date: 14 April 2001 * DOI:
https://doi.org/10.1038/sj.bdj.4800977 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
