Dietary restriction reduces angiogenesis and growth in an orthotopic mouse brain tumour model
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Diet and lifestyle produce major effects on tumour incidence, prevalence, and natural history. Moderate dietary restriction has long been recognised as a natural therapy that
improves health, promotes longevity, and reduces both the incidence and growth of many tumour types. Dietary restriction differs from fasting or starvation by reducing total food and caloric
intake without causing nutritional deficiencies. No prior studies have evaluated the responsiveness of malignant brain cancer to dietary restriction. We found that a moderate dietary
restriction of 30–40% significantly inhibited the intracerebral growth of the CT-2A syngeneic malignant mouse astrocytoma by almost 80%. The total dietary intake for the _ad_ _libitum_
control group (_n_=9) and the dietary restriction experimental group (_n_=10) was about 20 and 13 Kcal day−1, respectively. Overall health and vitality was better in the dietary
restriction-fed mice than in the _ad libitum_-fed mice. Tumour microvessel density (Factor VIII immunostaining) was two-fold less in the dietary restriction mice than in the _ad libitum_
mice, whereas the tumour apoptotic index (TUNEL assay) was three-fold greater in the dietary restriction mice than in the _ad libitum_ mice. CT-2A tumour cell-induced vascularity was also
less in the dietary restriction mice than in the _ad libitum_ mice in the _in vivo_ Matrigel plug assay. These findings indicate that dietary restriction inhibited CT-2A growth by reducing
angiogenesis and by enhancing apoptosis. Dietary restriction may shift the tumour microenvironment from a proangiogenic to an antiangiogenic state through multiple effects on the tumour
cells and the tumour-associated host cells. Our data suggest that moderate dietary restriction may be an effective antiangiogenic therapy for recurrent malignant brain cancers. SIMILAR
CONTENT BEING VIEWED BY OTHERS CA3 BRIDGES DIETARY RESTRICTION TO GLIOBLASTOMA SUPPRESSION AND TUMOR PROGRESSION AS A KEY DOWNSTREAM EFFECTOR Article Open access 28 May 2025 DEVELOPING
DIETARY INTERVENTIONS AS THERAPY FOR CANCER Article 25 May 2022 DAILY CALORIC RESTRICTION LIMITS TUMOR GROWTH MORE EFFECTIVELY THAN CALORIC CYCLING REGARDLESS OF DIETARY COMPOSITION Article
Open access 27 October 2021 MAIN About 35 000 people in the United States are diagnosed each year with primary or secondary brain tumours (Black, 1991). The prognosis for many of these
patients is poor despite new developments in neurosurgery, chemotherapy, and radiotherapy (Shapiro, 1999). Moreover, while the incidence of many cancers is decreasing, the incidence of brain
cancer is increasing in both children and the elderly (Lowry et al, 1998; Kaiser, 1999; McKinley et al, 2000). The highly infiltrative growth of malignant brain tumours and difficulties in
drug penetration of the neural parenchyma have limited therapeutic options. Hence, there is a crucial need for new and better brain tumour therapeutic strategies. Several studies suggest
that differences in diet and lifestyle can have major effects on tumour incidence, prevalence, and natural history (Blowers et al, 1997; Kaplan et al, 1997; Hu et al, 1999). Dietary
restriction (DR) has long been recognised as a natural therapy that improves health, promotes longevity, and significantly reduces both the incidence and growth of many tumour types (Rous,
1914; Tannenbaum, 1959; Weindruch and Walford, 1988; Birt et al, 1999; Kritchevsky, 1999b). Dietary restriction differs from severe fasting or starvation in that it reduces total caloric or
energy intake without causing deficiencies of any specific nutrients (Tannenbaum, 1959; Mukherjee et al, 1999a). The mechanisms by which DR reduces tumour growth are not yet clear, but
likely involve changes in tumour cells and in tumour associated host cells. Rous first suggested that DR might inhibit tumour growth by delaying host-mediated tumour vascularisation (Rous,
1914). Pili et al (1994) and Mukherjee et al (1999a) later provided direct support for Rous' hypothesis by showing that DR was antiangiogenic in experimental sarcomas and prostate
tumours, respectively. Moreover, the antiangiogenic effect of DR was observed whether the calories were derived from fats or carbohydrates suggesting that tumour angiogenesis may be more
sensitive to reductions in the amount rather than in the type of calories (Mukherjee et al, 1999a). Reduced total energy intake through DR may inhibit tumour growth by shifting tumour-host
cell interactions from a proangiogenic to an antiangiogenic state. Since neural tissues utilise glucose as the main energy substrate (Clarke and Sokoloff, 1999), brain tumours may be
responsive to dietary and nutritional therapies. Moreover, the reliance of brain tumours on glycolysis for energy should make them especially vulnerable to DR, as DR shifts energy metabolism
from glucose to ketone utilisation (Oudard et al, 1997; Greene et al, 2001). With the exception of an anecdotal report on the potential efficacy of a ketogenic diet toward paediatric
astrocytoma (Nebeling et al, 1995), no studies have been performed to our knowledge on the effects of DR as a therapeutic intervention for brain tumours. In this study, we show for the first
time that moderate DR can inhibit growth and vascularisation and enhance apoptosis in an orthotopic mouse brain tumour model. A preliminary account of these findings has appeared (Mukherjee
et al, 2001). MATERIALS AND METHODS MICE Mice of the C57BL/6J strain and the BALBc/J-SCID (severe combined immuno deficiency) strain were obtained from the Jackson laboratory (Bar Harbor,
ME, USA). The mice were propagated in the animal care facility of the department of Biology of Boston College, using animal husbandry conditions described previously (Flavin et al, 1991).
Male mice (8–10 weeks of age) were used for the studies and were provided with food either _ad libitum_ (AL) or under restricted conditions (as below). Water was provided AL to all mice. The
animal room was maintained at 22±1°C and cotton nesting pads were provided for additional warmth. All animal experiments were carried out with ethical committee approval in accordance with
the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Care Committee. Also, these procedures meet the standards required
by the UKCCCR guidelines (Workman et al, 1998). BRAIN TUMOUR MODEL The syngeneic CT-2A experimental mouse brain tumour used for these studies was generated in our laboratory after
implantation of 20-methylcholanthrene into the cerebral cortex of a C57BL/6J mouse according to the procedure of Zimmerman (Zimmerman and Arnold, 1941; Seyfried et al, 1992). Histologically,
the CT-2A brain tumour is broadly classified as a poorly differentiated highly malignant anaplastic astrocytoma (Seyfried et al, 1992). The tumour grows orthotopically as a soft,
noncohesive, and highly vascularised mass. INTRACEREBRAL TUMOUR IMPLANTATION The CT-2A tumour was implanted into the cerebral cortex of C57BL/6J mice using a trocar as we previously
described (Seyfried et al, 1987; Ranes et al, 2001). Briefly, mice were anaesthetised with pentobarbital (Vet Labs, Inc) intra-peritoneally and their heads were shaved and swabbed with 70%
ethyl alcohol under sterile conditions. Small CT-2A tumour pieces (about 1 mm3) from a C57BL/6J donor mouse were implanted into the right cerebral hemisphere of anaesthetised recipient mice
as we recently described (Ranes et al, 2001). All of the mice recovered from the surgical procedure and were returned to their cages when fully active. Initiation of tumours from intact
tumour pieces is preferable to initiation from cultured cells since the pieces already contain an established microenvironment that facilitates tumour growth. DIETARY RESTRICTION The mice
were group housed prior to the initiation of the experiment and were then separated and randomly assigned to either a control group that was fed AL or to an experimental group that was fed a
total DR of 30% (70% of the control group). Each mouse was housed singly in a plastic shoe box cage with a filter top and was given a cotton nesting pad for warmth. Dietary restriction was
initiated 7 days prior to tumour implantation and was continued for either 11 or 14 days after implantation. Total DR maintains a constant ratio of nutrients to energy, i.e., the average
daily food intake (grams) for the AL fed mice was determined every other day and the DR-fed mice were given 70% of that quantity on a daily basis (Mukherjee et al, 1999a). All mice received
PROLAB chow (Agaway Inc.), which contains a balance of mouse nutritional ingredients and, according to the manufacturer's specification, delivers 4.4 Kcal g−1 gross energy. Body weights
of all mice were recorded every other day. TUMOUR GROWTH Intracerebral tumour growth was analysed directly by measuring total tumour dry weight. Tumours were dissected from normal appearing
brain tissue, were frozen, and were then lyophilised to remove water. From our experience, total tumour dry weight is a more accurate measure of tumour growth than total wet weight because
individual CT-2A tumours can vary in the degree of haemorrhage and oedema. HISTOLOGY Tumour samples were fixed in 10% neutral buffered formalin (Sigma) and embedded in paraffin. Tumours were
sectioned at 5 um, stained with haematoxylin and eosin, and examined by light microscopy. FACTOR VIII STAINING AND MICROVESSEL QUANTITATION After deparaffinisation, rehydration, and
washing, the tumour sections were incubated with trypsin at 37°C for 30 min as we recently described for prostate tumours (Mukherjee et al, 1999a). Briefly, the sections were quenched with
0.3% H2O2-methanol for 30 min and then blocked with 10% normal goat serum in PBA buffer (100 ml of 0.01 M phosphate buffer with 0.9% sodium chloride, and 1.0 g bovine serum albumin and 0.1
ml Tween 20, pH 7.4). The sections were treated with rabbit polyclonal antibody directed against human factor VIII-related antigen (Dako Corp., Carpinteria, CA, USA; 1:100 dilution with PBA)
followed by a biotinylated anti-rabbit IgG at 1:100 dilution (Vector Laboratories, Inc., Burlingame, CA, USA). The sections were then treated with avidin-biotin complex followed by 3-3′
diaminobenzidine as substrate for staining according to the manufacturer's directions (Vectastain Elite ABC kit; Vector Laboratories, Inc.). The sections were then rinsed three times
with PBS (0.01 M phosphate buffer with 0.9% NaCl). Sections were counter stained with methyl green and mounted. Corresponding tissue sections without primary antibody served as negative
controls. Microvessel density was quantified by examining areas of vascular hotspots as previously described by Weidner et al (1991) with some modifications. Sections were scanned at low
magnification (40 × and 100 ×) for the localisation of vascular hotspots. The three most vascular areas of the tumour, not containing necrosis, were determined and then counted at higher
magnification (200 ×). The values of the three sections were averaged and the results of three independent CT-2A tumours were analyzed. Branching structures were counted as a single vessel
as previously shown (Mukherjee et al, 1999a). _IN SITU_ APOPTOTIC CELL DETECTION (TUNEL) Apoptotic cells were detected using the ApopTag _in situ_ detection kit TUNEL (terminal
deoxynucleotidyl transferase mediated deoxyuridine triphosphate biotin nick end labelling) (Oncor, Gaithersberg, MD, USA) as we previously described (Mukherjee et al, 1999a). After
deparaffinisation, rehydration and washing in PBS, the tissue sections were treated with proteinase K (20 μg ml−1) for 15 min at room temperature and were then washed in PBS. The sections
were treated with 3% H2O2 in PBS for 5 min to quench endogenous peroxide activities. The 3′ hydroxy DNA strand breaks were enzymatically labelled with digoxygeninnucleotide via TdT and
incubated for 1 h at 37°C. The reaction was terminated with stop buffer according to the manufacturer's protocol. Sections were then treated with anti-degoxygenin peroxidase for 30 min
at room temperature, washed, stained with 3-3′ diaminobenzidine substrate, counter stained with hematoxylin, and finally were mounted. Tissue sections of post weaning normal female mouse
mammary glands, provided by Oncor, were used as a positive control and staining of a corresponding tissue section without added TdT served as the negative control. The apoptotic index was
expressed as AI%=A × 100/(A+C), where A=TUNEL positive cells and C=counter stained unlabelled cells. The tumour sections were scanned at lower magnification (40 × and 100 ×) for nonnecrotic
areas and approximately 2000 total cells were counted for each section at higher magnification (400 ×). The values of the three sections were averaged and the results of three independent
CT-2A tumours were analysed. PROLIFERATION INDEX Proliferation index measured the fraction of cells with proliferating cell nuclear antigen (PCNA) staining as we previously described
(Mukherjee et al, 1999a). After deparaffinisation, rehydration and washing, the tissue sections were soaked in 10 mM citrate buffer (pH 6.0). The sections were heated in a microwave oven for
15 min (defrost cycle) and then cooled to room temperature to unmask the PCNA. Sections were then stained by the same procedures as described above except we used 10% horse serum as
blocking agent and PCNA mouse monoclonal antibody (Dako) as the primary antibody. Light microscopy (400 ×) was used to count both PCNA positive proliferating cells and total tumour cells in
three non necrotic areas of each tissue section as previously shown (Mukherjee et al, 1999a). _IN VIVO_ MATRIGEL MODEL OF ANGIOGENESIS Male BALB/c-SCID mice were divided into two groups of
three mice each: a control AL group and a 30% DR group. Dietary restriction treatment was initiated 7 days prior to tumour cell injection. CT-2A tumour cells were grown in culture and
harvested with 0.25% trypsin containing 1 mM EDTA. The cells were washed twice, resuspended in serum free DMEM, and then thoroughly mixed with Matrigel (Collaborative Biomedical) 1:2 (v v−1)
at 4°C as we recently described (Manfredi et al, 1999). Mice were anaesthetised with Isovet (Schering Plough Animal Health, Omaha, NE, USA) and then injected with 1 × 106 cells in 300 μl of
Matrigel subcutaneously in the dorsal midline using a prechilled tuberculin syringe (27 gauge needle). The mice were maintained for another 7 days under the dietary regime at which time
they were euthanised and the Matrigel plug with the surrounding skin was removed as we previously described (Manfredi et al, 1999). Vascularity was photographed using a dissecting
photomicroscope (Leica, WILD macroskop). RESULTS No adverse effects were seen in the mice maintained on the 30–40% DR. Despite a reduction in total body weight, the DR-fed mice appeared
healthy and were more active than the AL-fed mice as assessed by ambulatory and grooming behaviour. No signs of vitamin or mineral deficiency were observed in the DR-fed mice according to
standard criteria for mice (Hoag and Dickie, 1968). These findings are consistent with the well-recognized health benefits of mild to moderate diet restriction in rodents (Weindruch and
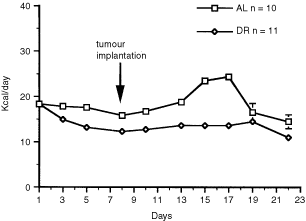
Walford, 1988; Keenan et al, 1999). ENERGY INTAKE Our experimental design involved pretreatment with DR for 7 days prior to intracerebral tumour implantation. This was done to separate the
physiological stress of single cage housing and food restriction from surgical brain trauma. A slight reduction in energy intake was noticed in both the AL-fed and the DR-fed mice at the
initiation of the experiment (Figure 1). This was attributed to the effects of moving the mice from group housing to single cage housing. Energy intake increased significantly in the AL-fed
group about 2 days after intracerebral tumour implantation. This resulted from a period of hyperphagia likely associated with cerebral hyperglycolysis following the traumatic injury of
tumour implantation (Bergsneider et al, 1997). Energy intake was not increased in the DR-fed mice to compensate for hyperphagia. Consequently, energy intake was indirectly reduced in the
DR-fed mice from 30% to about 40% of that in the control AL-fed group. Hyperphagia associated with orthotopic brain tumour growth is a novel finding and was not observed in C57BL/6J mice
with the CT-2A tumour grown subcutaneously in flank (Ranes et al, 2001). The total energy intake of the AL-fed group was about 18 Kcal day−1, but rose to about 24 Kcal day−1 during the
hyperphagic period (Figure 1). The total energy intake of the DR-fed group was adjusted to 13 Kcal day−1 during the 22 day experiment. The DR-fed mice lost about 12% of their body weight
during the first week of treatment and their weights remained significantly lower than those of the AL group throughout the study. The mean (±s.e.m.) body weights (g) of the AL and DR mice
after 17 days of treatment were 23.6±0.6 and 20.3±0.4, respectively (_P_<0.01, two tailed _t_-test). Total energy intake and body weights dropped after 17 days in the AL-fed group due to
increased tumour burden. DR REDUCED INTRACEREBRAL CT-2A TUMOUR GROWTH Dry weights of the intracerebral CT-2A tumours were approximately 79.5% lower in the DR-fed mice than in the AL-fed mice
(Figure 2). It is important to mention that all implanted tumours grew in both the AL and DR groups. These findings indicate that DR did not prevent tumour take, but significantly reduced
intracerebral growth of the malignant CT-2A brain tumour. We do not think the reduced food intake beyond day 19 in the control AL mice reduced the difference in tumor size between the AL and
CR mice. INFLUENCE OF DR ON TUMOUR VASCULARITY, APOPTOSIS, AND CELL PROLIFERATION We next examined tumour morphology and blood vessel densities using H&E staining and Factor VIII
immunostaining to determine if DR influenced tumour angiogenesis. Three independent tumours from the AL and DR groups were chosen at random for these studies. The number and size of blood
vessels and tumour cell density were noticeably less in the DR-fed mice than in the AL-fed mice (Figure 3A,B). Also, the tumour microvessel density of the DR-fed mice was about half of that
in the AL-fed mice (Figure 3C,D, and Table 1). To determine if DR influenced programmed cell death (apoptosis) in the CT-2A tumour, we compared the number of TUNEL positive cells (apoptotic
index) in the AL-fed and DR-fed mice. The apoptotic index was almost three-fold greater in the DR mice than in the AL mice (Figure 3E,F and Table 1). No significant difference was found,
however, between the DR and AL mice for the PCNA proliferation index (Table 1), suggesting that the DR-induced reduction of CT-2A growth was not associated with reduced tumour cell
proliferation. DR REDUCED VASCULARITY IN THE _IN VIVO_ MATRIGEL MODEL OF ANGIOGENESIS The _in vivo_ Matrigel angiogenesis model represents early events of angiogenesis and tumour progression
and is dependent on activation and infiltration of host stromal cells which include monocytes, macrophages, and endothelial cell precursors (Manfredi et al, 1999). DR reduced vascularity
when the CT-2A tumour cells were grown in the _in vivo_ Matrigel model of angiogenesis (Figure 4). Although blood vessel quantitation is difficult in the plugs, it is clear from the figure
that both the number and dilation of vessels was noticeably less in and around the plugs from the DR-fed mice than from the AL-fed mice. Similar qualitative differences were seen in the
other independent sample. These findings indicate that DR reduces the angiogenic properties of the CT-2A tumour cells whether grown within or outside of the central nervous system.
DISCUSSION We found that a moderate DR of 30–40% significantly reduced angiogenesis and growth of the CT-2A experimental mouse astrocytoma. Moreover, DR enhanced CT-2A cell apoptosis without
effecting cell proliferation. Previous studies showed that moderate DR could reduce the growth of histologically diverse non-neural tumours (Rous, 1914; Tannenbaum, 1959; Kritchevsky,
1999a; Mukherjee et al, 1999a). Our studies are the first to document this phenomenon in a brain tumour model and suggest that brain tumours may be especially vulnerable to the
growth-inhibitory effects of DR. It will be important to document the extent to which DR reduces angiogenesis and growth in other brain tumour models. Despite a 12% reduction in body weight,
the DR-fed mice were more active and healthy than the AL fed mice. Keenan and co-workers recently suggested that the AL feeding of sedentary rodents is a form of over feeding that can
produce adverse health effects (Keenan et al, 1999). Our results support this contention since CT-2A tumour angiogenesis and growth was significantly greater in mice under AL feeding than
under DR. We found that angiogenic biomarkers may be useful for evaluating the influence of energy intake and nutrition on the growth and progression of experimental brain cancer. Moderate
DR significantly reduced microvessel density, increased the apoptotic index, but had little effect on the PCNA labelling index in the CT-2A brain tumour. Other investigators have also
reported that antiangiogenic growth factors and cytokines can reduce tumour microvessel density, increase apoptosis, but have little effect on cell proliferation (Holmgren et al, 1995;
O'Reilly et al, 1996; Tanaka et al, 1997; Beecken et al, 2001). Our results therefore support previous findings that DR produces a pattern of biomarker changes similar to the changes
seen following the implementation of antiangiogenic therapies (Mukherjee et al, 1999a; 1999b). The mechanisms by which DR reduced CT-2A tumour angiogenesis and growth are not yet clear, but
may involve effects on both the tumour cells and the tumour-associated host cells. It is documented that human and experimental gliomas are dependent on glycolysis for energy (Mies et al,
1990; Ikezaki et al, 1992; Oudard et al, 1997), and that DR-induced caloric restriction reduces glycolytic energy and down-regulates glycolytic gene expression (Lee et al, 2000; Cao et al,
2001; Greene et al, 2001). Additionally, the DR-induced down regulation of glycolysis should also reduce the level of pyruvic acid, a glycolytic end product with angiogenic activity (Lee et
al, 2001). Glucose is used exclusively for adult brain energy metabolism under normal physiological conditions, but the brain will metabolise ketone bodies for energy when blood glucose
levels decrease as during fasting or DR (Clarke and Sokoloff, 1999; Greene et al, 2001). Since ketone bodies are metabolised directly to acetyl-CoA in the mitochondria, they bypass
cytoplasmic glycolysis and provide energy directly through the Krebs cycle (Nehlig and Pereira de Vasconcelos, 1993; Clarke and Sokoloff, 1999). We recently showed that DR produces ketosis
in epileptic mice and that the degree of ketosis is inversely proportional to blood glucose levels (Greene et al, 2001). Further studies will be needed to determine if reduced glycolytic
energy and elevated ketosis underlie the antiangiogenic and growth inhibitory effects of DR. In addition to possible effects on energy metabolism, DR may also reduce CT-2A angiogenesis and
growth through effects on tumour associated host cells. The progression of human and experimental brain tumours is dependent to a large extent on the proangiogenic and inflammatory
properties of activated glia and macrophages (Seyfried, 2001). Indeed, the degree of tumour angiogenesis and malignancy is generally correlated with the number and activation state of
tumour-associated macrophages and microglia (Wood and Morantz, 1979; Roggendorf et al, 1996; Nishie et al, 1999; Polverini, 1999; Badie and Schartner, 2000). Recent studies also indicate
that moderate DR reduces brain inflammation associated with ageing and neurodegeneration (Duan et al, 2001; Lee et al, 2000). Furthermore, dietary energy restriction can elevate
glucocorticoid hormone that could further reduce tumour inflammation and growth through down regulation of stress-activated protein kinase pathways (Birt et al, 1999). Hence, DR may reduce
CT-2A progression through a global down-regulation of inflammatory and angiogenic properties of the tumour microenvironment. We also found that DR caused a noticeable reduction in the number
and the dilation of blood vessels in the _in vivo_ Matrigel model of angiogenesis indicating that DR can reduce angiogenesis both within and outside of the central nervous system. It is
possible that DR reduces the inflammatory properties of tumour-associated host cells and thereby shifts tumour-host cell interactions from a proangiogenic to an antiangiogenic state. Studies
are planned to test these possibilities. Our findings may have relevance to those _in vivo_ studies where food intake and body weight are reduced in conjunction with anticancer therapies or
with cancer cachexia. Reduction of energy intake as a covariable of anorexic anticancer therapies may confound interpretation of results (Ranes et al, 2001). It would be important therefore
to control for the antitumour effects of dietary reduction in the preclinical evaluation of new cancer drugs. Weight loss associated with cancer cachexia differs from weight loss associated
with anorexia (reduction in food intake) since cachexia can occur without anorexia and is produced from factors released by the tumour (Tisdale, 2001). Although appearing counterintuitive,
we suggest that DR may antagonise cachexia by reducing tumour size and therby reducing levels of procachexic factors. Although DR is recognised as a preventative measure for carcinogenisis,
it is clear from our findings on the the CT-2A brain tumour that DR is not a preventative intervention since all of the tumours implanted grew despite the 7 day DR pretreatment period. The
DR-induced inhibition of CT-2A angiogenesis and growth suggests that DR retards tumour progression. Whether DR would also increase the survival time of CT-2A-tumour bearing mice is not
clear. Survival studies are difficult with this rapidly growing brain tumour model since the tumour will grow through the implantation burr hole and then subcutaneously over the skull as we
previously described for the EPEN model (Seyfried et al, 1987). This relieves intracranial pressure and artificially extends longevity. In humans with malignant brain tumours, it is the
intracranial pressure that usually leads to morbidity. In summary, we have demonstrated that DR alone can reduce angiogenesis and growth in an experimental mouse brain tumour. Moreover, the
antitumour action of DR likely operates through multiple effects on the tumour cells and on the tumour associated host cells. We contend that our experimental protocol may have therapeutic
potential for recurrent human gliomas since the time of surgical tumour resection in humans would be comparable to the time of tumour transplantation in mice. In other words, implementation
of DR in the clinic could be most effective immediately following tumour removal and may delay tumour recurrence. Because DR is easy to administer and is devoid of adverse side effects, our
preclinical studies suggest that DR or caloric restriction may have efficacy as a non-invasive therapy for recurrent malignant brain cancers. CHANGE HISTORY * _ 16 NOVEMBER 2011 This paper
was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Badie B, Schartner JM (2000) Flow cytometric
characterization of tumor-associated macrophages in experimental gliomas. _Neurosurgery_ 46: 957–961 CAS Google Scholar * Beecken WD, Fernandez A, Joussen AM, Achilles EG, Flynn E, Lo KM,
Gillies SD, Javaherian K, Folkman J, Shing Y (2001) Effect of antiangiogenic therapy on slowly growing, poorly vascularized tumors in mice. _J Natl Cancer Inst_ 93: 382–387 Article CAS
Google Scholar * Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, Phelps ME, McArthur DL, Caron MJ, Kraus JF, Becker DP (1997) Cerebral hyperglycolysis following severe
traumatic brain injury in humans: a positron emission tomography study. _J Neurosurg_ 86: 241–251 Article CAS Google Scholar * Birt DF, Yaktine A, Duysen E (1999) Glucocorticoid mediation
of dietary energy restriction inhibition of mouse skin carcinogenesis. _J Nutr_ 129: 571S–574S Article CAS Google Scholar * Black PM (1991) Brain tumors. Part 1. _N Engl J Med_ 324:
1471–1476 Article CAS Google Scholar * Blowers L, Preston-Martin S, Mack WJ (1997) Dietary and other lifestyle factors of women with brain gliomas in Los Angeles County (California, USA).
_Cancer Causes Control_ 8: 5–12 Article CAS Google Scholar * Cao SX, Dhahbi JM, Mote PL, Spindler SR (2001) Genomic profiling of short- and long-term caloric restriction effects in the
liver of aging mice. _Proc Natl Acad Sci USA_ 98: 10630–10635 Article CAS Google Scholar * Clarke DD, Sokoloff L (1999) Circulation and energy metabolism in the brain. In_Basic
Neurochemistry_ Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD (eds) pp637–669, New York: Lippincott-Raven Google Scholar * Duan W, Lee J, Guo Z, Mattson MP (2001) Dietary
restriction stimulates BDNF production in the brain and thereby protects neurons against excitotoxic injury. _J Mol Neurosci_ 16: 1–12 Article CAS Google Scholar * Flavin HJ, Wieraszko A,
Seyfried TN (1991) Enhanced aspartate release from hippocampal slices of epileptic (El) mice. _J Neurochem_ 56: 1007–1011 Article CAS Google Scholar * Greene AE, Todorova MT, McGowan R,
Seyfried TN (2001) Caloric restriction inhibits seizure susceptibility in epileptic EL mice by reducing blood glucose. _Epilepsia_ 42: 1371–1378 Article CAS Google Scholar * Hoag WG,
Dickie MM (1968) Nutrition. In_Biology of the Laboratory Mouse_ Green EL (ed) New York: Dover Google Scholar * Holmgren L, O'Reilly MS, Folkman J (1995) Dormancy of micrometastases:
balanced proliferation and apoptosis in the presence of angiogenesis suppression. [see comments]_Nat Med_ 1: 149–153 Article CAS Google Scholar * Hu J, La Vecchia C, Negri E, Chatenoud L,
Bosetti C, Jia X, Liu R, Huang G, Bi D, Wang C (1999) Diet and brain cancer in adults: a case-control study in northeast China. _Int J Cancer_ 81: 20–23 Article CAS Google Scholar *
Ikezaki K, Black KL, Conklin SG, Becker DP (1992) Histochemical evaluation of energy metabolism in rat glioma. _Neurol Res_ 14: 289–293 Article CAS Google Scholar * Kaiser J (1999) No
meeting of minds on childhood cancer. _Science_ 286: 1832–1834 Article CAS Google Scholar * Kaplan S, Novikov I, Modan B (1997) Nutritional factors in the etiology of brain tumors:
potential role of nitrosamines, fat, and cholesterol. _Am J Epidemiol_ 146: 831–832 Article Google Scholar * Keenan KP, Ballam GC, Soper KA, Laroque P, Coleman JB, Dixit R (1999) Diet,
caloric restriction, and the rodent bioassay. _Toxicol Sci_ 52: 24–34 Article CAS Google Scholar * Kritchevsky D (1999a) Caloric restriction and experimental carcinogenesis. _Toxicol Sci_
52: 13–16 Article CAS Google Scholar * Kritchevsky D (1999b) Fundamentals of nutrition: applications to cancer research. In_Nutritional Oncology_ Heber D, Blackburn GL, Go VLW (eds)
pp5–10, Boston: Academic Press Google Scholar * Lee CK, Weindruch R, Prolla TA (2000) Gene-expression profile of the ageing brain in mice. _Nat Genet,_ 25: 294–297 Article CAS Google
Scholar * Lee MS, Moon EJ, Lee SW, Kim MS, Kim KW, Kim YJ (2001) Angiogenic activity of pyruvic acid in _in vivo_ and _in vitro_ angiogenesis models. _Cancer Res_ 61: 3290–3293 CAS PubMed
Google Scholar * Lowry JK, Snyder JJ, Lowry PW (1998) Brain tumors in the elderly: recent trends in a Minnesota cohort study. _Arch Neurol_ 55: 922–928 Article CAS Google Scholar *
Manfredi MG, Lim S, Claffey KP, Seyfried TN (1999) Gangliosides influence angiogenesis in an experimental mouse brain tumor. _Cancer Res_ 59: 5392–5397 CAS PubMed Google Scholar *
McKinley BP, Michalek AM, Fenstermaker RA, Plunkett RJ (2000) The impact of age and sex on the incidence of glial tumors in New York state from 1976 to 1995. _J Neurosurg_ 93: 932–939
Article CAS Google Scholar * Mies G, Paschen W, Ebhardt G, Hossmann KA (1990) Relationship between of blood flow, glucose metabolism, protein synthesis, glucose and ATP content in
experimentally-induced glioma (RG1 2.2) of rat brain. _J Neurooncol_ 9: 17–28 Article CAS Google Scholar * Mukherjee P, El-Abbadi MM, Kasperzyk JL, Seyfried TN (2001) Caloric restriction
reduces growth and angiogenesis in a mouse brain tumor. _Proc Amer Assoc Cancer Res_ 42: 651–652 Google Scholar * Mukherjee P, Sotnikov AV, Mangian HJ, Zhou JR, Visek WJ, Clinton SK (1999a)
Energy intake and prostate tumor growth, angiogenesis, and vascular endothelial growth factor expression. _J Natl Cancer Inst_ 91: 512–523 Article CAS Google Scholar * Mukherjee P, Zhau
J-R, Sotnikov AV, Clinton SK (1999b) Dietary and nutritional modulation of tumor angiogenesis. In_Antiangiogenic Agents in Cancer Therapy_ Teicher BA (ed) pp237–261, Totowa, NJ: Humana Press
Chapter Google Scholar * Nebeling LC, Miraldi F, Shurin SB, Lerner E (1995) Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case
reports. _J Am Coll Nutr_ 14: 202–208 Article CAS Google Scholar * Nehlig A, Pereira de Vasconcelos A (1993) Glucose and ketone body utilization by the brain of neonatal rats. _Prog
Neurobiol_ 40: 163–221 Article CAS Google Scholar * Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, Ito Y, Inamura T, Ikezaki K, Fukui M, Iwaki T, Kuwano M (1999) Macrophage
infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. _Clin Cancer Res_ 5: 1107–1113 CAS PubMed Google Scholar * O'Reilly MS, Holmgren L, Chen C,
Folkman J (1996) Angiostatin induces and sustains dormancy of human primary tumors in mice. _Nat Med_ 2: 689–692 Article CAS Google Scholar * Oudard S, Boitier E, Miccoli L, Rousset S,
Dutrillaux B, Poupon MF (1997) Gliomas are driven by glycolysis: putative roles of hexokinase, oxidative phosphorylation and mitochondrial ultrastructure. _Anticancer Res_ 17: 1903–1911 CAS
PubMed Google Scholar * Pili R, Guo Y, Chang J, Nakanishi H, Martin GR, Passaniti A (1994) Altered angiogenesis underlying age-dependent changes in tumor growth. _J Natl Cancer Inst_ 86:
1303–1314 Article CAS Google Scholar * Polverini PJ (1999) Contribution of the extracellular matrix and macrophages in angiogenesis. In_Antiangiogenic Agents in Cancer Therapy_ Teicher
BA (ed) pp65–75, Totowa, New Jersey: Humana Press Chapter Google Scholar * Ranes MK, El-Abbadi M, Manfredi MG, Mukherjee P, Platt FM, Seyfried TN (2001) N -butyldeoxynojirimycin reduces
growth and ganglioside content of experimental mouse brain tumours. _Br J Cancer_ 84: 1107–1114 Article CAS Google Scholar * Roggendorf W, Strupp S, Paulus W (1996) Distribution and
characterization of microglia/macrophages in human brain tumors. _Acta Neuropathol_ 92: 288–293 Article CAS Google Scholar * Rous P (1914) The influence of diet on transplanted and
spontaneous mouse tumors. _J Exp Med_ 20: 433–451 Article CAS Google Scholar * Seyfried TN (2001) Perspectives on brain tumor formation involving macrophages, glia, and neural stem cells.
_Perspect Biol Med_ 44: 263–282 Article CAS Google Scholar * Seyfried TN, El-Abbadi M, Roy ML (1992) Ganglioside distribution in murine neural tumors. _Mol Chem Neuropathol_ 17: 147–167
Article CAS Google Scholar * Seyfried TN, Yu RK, Saito M, Albert M (1987) Ganglioside composition of an experimental mouse brain tumor. _Cancer Res_ 47: 3538–3542 CAS PubMed Google
Scholar * Shapiro WR (1999) Current therapy for brain tumors: back to the future. _Arch Neurol_ 56: 429–432 Article CAS Google Scholar * Tanaka T, Manome Y, Wen P, Kufe DW, Fine HA
(1997) Viral vector-mediated transduction of a modified platelet factor 4 cDNA inhibits angiogenesis and tumor growth. _Nat Med_ 3: 437–442 Article CAS Google Scholar * Tannenbaum A
(1959) Nutrition and cancer. In_Physiopathology of Cancer_ Homburge F (ed) pp517–562, NY: Paul B Hober Google Scholar * Tisdale MJ (2001) Cancer anorexia and cachexia. _Nutrition_ 17:
438–442 Article CAS Google Scholar * Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis – correlation in invasive breast carcinoma. _N Engl J Med_ 324: 1–8
Article CAS Google Scholar * Weindruch R, Walford RL (1988) _The retardation of aging and disease by dietary restriction._ Springfield, IL: Thomas Google Scholar * Wood GW, Morantz RA
(1979) Immunohistologic evaluation of the lymphoreticular infiltrate of human central nervous system tumors. _J Natl Cancer Inst_ 62: 485–491 Article CAS Google Scholar * Workman P,
Twentyman P, Balkwill F, Balmain A, Chaplin D, Double J, Embleton J, Newell D, Raymond R, Stables J, Stephens T, Wallace J (1998) United Kingdom Co-ordinating Committee on Cancer Research
(UKCCCR) Guidelines for the Welfare of Animals in Experimental Neoplasia (Second Edition). _Br J Cancer_ 77: 1–10 Google Scholar * Zimmerman HM, Arnold H (1941) Experimental brain tumors:
I. tumors produced with methylcholanthrene. _Cancer Res_ 1: 919–938 CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported in part from NIH grant (HD39722), The
Boston College Research Expense Fund, and a grant from the American Institute of Cancer Research. We would like to thank Dr Grant Balkema and the Dana-Farber/Harvard Cancer Center Pathology
Core Facilities for technical assistance. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Biology Department, Boston College, Chestnut Hill, Massachusetts, 02467, MA, USA P Mukherjee, M M
El-Abbadi, J L Kasperzyk, M K Ranes & T N Seyfried Authors * P Mukherjee View author publications You can also search for this author inPubMed Google Scholar * M M El-Abbadi View author
publications You can also search for this author inPubMed Google Scholar * J L Kasperzyk View author publications You can also search for this author inPubMed Google Scholar * M K Ranes View
author publications You can also search for this author inPubMed Google Scholar * T N Seyfried View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHOR Correspondence to T N Seyfried. RIGHTS AND PERMISSIONS From twelve months after its original publication, this work is licensed under the Creative Commons
Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Mukherjee, P., El-Abbadi, M., Kasperzyk, J. _et al._ Dietary restriction reduces angiogenesis and growth in an orthotopic mouse brain tumour model. _Br J Cancer_
86, 1615–1621 (2002). https://doi.org/10.1038/sj.bjc.6600298 Download citation * Received: 17 December 2001 * Revised: 04 March 2002 * Accepted: 11 March 2002 * Published: 20 May 2002 *
Issue Date: 20 May 2002 * DOI: https://doi.org/10.1038/sj.bjc.6600298 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry,
a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * glioma * glycolysis *
inflammation * energy metabolism * caloric restriction * microenvironment
