Interleukin-2/interferon-α2a/13-retinoic acid-based chemoimmunotherapy in advanced renal cell carcinoma: results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN)
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

We performed a prospectively randomised clinical trial to compare the efficacy of four subcutaneous interleukin-2-(sc-IL-2) and sc interferon-α2a (sc-IFN-α2a)-based outpatient regimens in
379 patients with progressive metastatic renal cell carcinoma. Patients with lung metastases, an erythrocyte sedimentation rate ⩽70 mm h−1 and neutrophil counts ⩽6000 μl−1 (group I) were
randomised to arm A: sc-IL-2, sc-IFN-α2a, peroral 13-cis-retinoic acid (po-13cRA) (n=78), or arm B: arm A plus inhaled-IL-2 (n=65). All others (group II) were randomised to arm C: arm A plus
intravenous 5-fluorouracil (iv-5-FU) (n=116), or arm D: arm A plus po-Capecitabine (n=120). Median overall survival (OS) was 22 months (arm A; 3-year OS: 29.7%) and 18 months (arm B; 3-year
OS: 29.2%) in group I, and 18 months (arm C; 3-year OS: 25.7%) and 16 months (arm D; 3-year OS: 32.6%) in group II. There were no statistically significant differences in OS,
progression-free survival, and objective response between arms A and B, and between arms C and D, respectively. Given the known therapeutic efficacy of sc-IL-2/sc-INF-α2a/po-13cRA-based
outpatient chemoimmunotherapies, our results did not establish survival advantages in favour of po-Capecitabine vs iv-5-FU, and in favour of short-term inhaled-IL-2 in patients with advanced
renal cell carcinoma receiving systemic cytokines.
The prognosis of metastatic renal carcinoma remains poor. Although this tumour is highly resistant to chemotherapy and hormone therapy, promising results have been reported with the use of
molecular agents that is, recombinant cytokines, notably recombinant interleukin-2 (IL-2) and interferon-α (IFN-α), given intravenously, subcutanously alone or in combination in outpatient
regimens with objective response rates between 6 and 31% (Rosenberg et al, 1987; Atzpodien et al, 1990; Sleijfer et al, 1992; Jayson et al, 1998; Tourani et al, 2003).
When the present trial was planned, various studies focused on the combination of immunmodulator substances and chemotherapeutic agents to increase antitumour activity. In preliminary
reports on oral 13-cis-retinoic acid (po-13cRA), a cell differentiation regulator, po-13cRA could enhance antitumour efficacy in IL-2/IFN-α- or chemoimmunotherapy-treated metastatic renal
cell carcinoma patients, with objective response rates between 17 and 42% (Atzpodien et al, 1995; Stadler et al, 1998). In the presence of pulmonary metastases, locoregional administration
of inhaled-IL-2 was reported to yield low toxicity combined with objective response rates of pulmonary disease of 2.5–21% (Lorenz et al, 1996; Merimsky et al, 2004). Other reports showed
that the combination of cytokines with intravenous 5-fluorouracil (iv-5-FU) could increase objective response rates to between 12 and 39% (van Herpen et al, 2000; Atzpodien et al, 2001,
2004). Preliminary results of a phase II study combining IL-2/IFN-α with oral Capecitabine, which is converted to 5-FU in vivo, reported objective response rates of 34% (Oevermann et al,
2000).
Here, we prospectively compared the long-term therapeutic efficacy of four outpatient combination regimens: arm A (sc-IL-2, sc-IFN-α2a, po-13cRA) and arm B (arm A plus inhaled-IL-2) in
patients with pulmonary disease, and arm C (arm A plus iv-5-FU) and arm D (arm A plus po-Capecitabine) in all others.
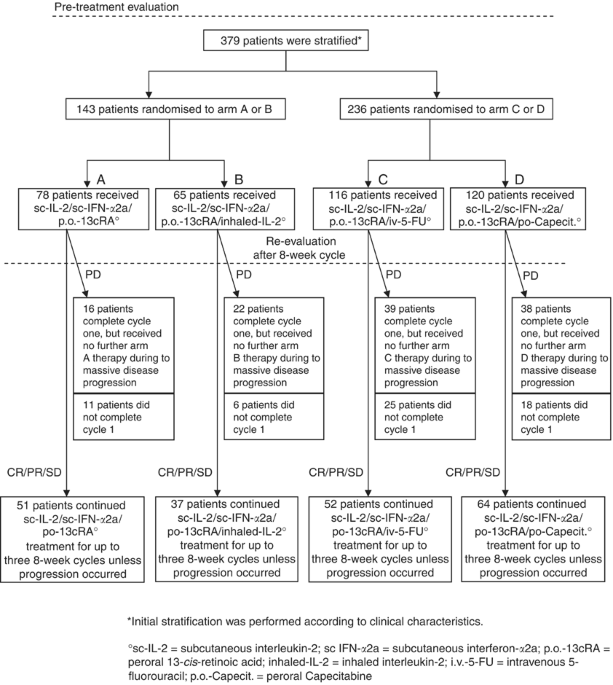
Three hundred and seventy-nine patients with metastastic renal cell carcinoma were stratified into two groups (Figure 1). Group I patients (n=143) were subsequently randomised to arm A
(sc-IL-2, sc-IFN-α2a, po-13-cRA) or arm B (arm A plus inhaled-IL-2), whereas group II patients (all others; n=236) were randomised to arm C (arm A plus iv-5-FU) or arm D (arm A plus
po-Capecitabine). Median follow-up of these patients was 18 months (range 0–83 months). Patient pretreatment included radical tumour nephrectomy (n=343), radiotherapy (n=51), chemotherapy
(n=11), immunotherapy (n=18), chemoimmunotherapy (n=19), naturopathic therapy (n=2), and others (n=7) (Table 1).
Criteria for entry into the trial were as follows: histologically confirmed progressive and irresectable metastatic renal cell carcinoma; an expected survival duration of more than 3 months;
Karnofsky performance status >80%; age between 18 and 80 years; white blood cell count >3500 μl−1; platelet count >100 000 μl−1; haematocrit >30%; serum bilirubin, and creatinine
