The proportion of tumour cells is an independent predictor for survival in colorectal cancer patients
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT BACKGROUND: The proportion of epithelial and stromal cells in tumours is thought to have an important role in the progression of epithelial malignancy. We aimed to determine whether
the relative proportion of tumour (PoT) was related to survival in colorectal cancer. METHODS: The PoT at the luminal surface was measured by point counting using virtual tissue sections in
a series of 145 colorectal cancer cases. The relationship of PoT to clinicopathological parameters including cancer-specific survival was analysed. Modified receiver operating
characteristic curves were used to determine the optimum cut off points to dichotomise the data for survival analyses. RESULTS: Tumours with PoT-low (⩽47%) were associated with significantly
lower cancer-specific survival when compared to PoT-high (hazard ratio (HR)=2.087, 95% CI=1.088–4.003, _P_=0.024). On sub-analysis, the prognostic effect remained significant in colonic
tumours (HR=2.474, 95% CI=1.132–5.408, _P_=0.019) and tumour, node, metastasis stage III disease (HR=3.480, 95% CI=0.325–9.136, _P_=0.007). Multivariate Cox regression analysis demonstrated
that PoT was an independent prognostic marker when adjusted for age, T stage, N stage and extramural vascular invasion (_P_=0.017). CONCLUSION: This study suggests that a low proportion of
tumour cells in colorectal cancer is related to poor cancer-specific survival. A relatively quick, inexpensive and well-established method such as point counting on diagnostic tissue
sections could be used to identify a subset of patients who may benefit from adjuvant therapy. SIMILAR CONTENT BEING VIEWED BY OTHERS PROGNOSTIC SCORING SYSTEM BASED ON INDICATORS REFLECTING
THE TUMOR GLANDULAR DIFFERENTIATION AND MICROENVIRONMENT FOR PATIENTS WITH COLORECTAL CANCER Article Open access 20 June 2024 DEVELOPMENT AND VALIDATION OF NOMOGRAMS FOR PREDICTING OVERALL
SURVIVAL AND CANCER-SPECIFIC SURVIVAL IN UNRESECTED COLORECTAL CANCER PATIENTS UNDERGOING CHEMOTHERAPY Article Open access 11 April 2025 TUMOUR BUDDING AND ITS CLINICAL IMPLICATIONS IN
GASTROINTESTINAL CANCERS Article Open access 30 June 2020 MAIN Colorectal cancer (CRC) is a common disease and despite significant advances in its treatment, the 5-year overall survival
remains around 50% (Cancer Research UK, 2009). Recent figures show that CRC is now the second commonest cause of cancer related mortality in the United Kingdom with over 16 000 deaths
occurring annually (Cancer Research UK, 2009). Staging systems such as Dukes (Gabriel et al, 1935) and tumour, node, metastasis (TNM) (Sobin and Wittekind, 1997, pp 66–69) are routinely used
to predict prognosis following surgery, however, patients diagnosed at the same stage of disease often have markedly different outcomes (Morris et al, 2007). Current research aims to
identify additional prognostic markers that can be used to stratify CRC patients and identify those which may benefit from adjuvant therapy. Similar to all other malignant epithelial
tumours, CRC is composed of carcinoma cells admixed with stromal fibroblasts, lymphatic and vascular channels, and inflammatory cells, often referred to as the tumour microenvironment. This
microenvironment is becoming increasingly recognised as having an important role in tumour cell invasion and the ability to metastasise (De Wever and Mareel, 2003). Very few studies in
selected cancer subtypes, such as breast (Baak et al, 1985), lung (Nakajima, 1991; Maeshima et al, 2002; Das Neves Pereira et al, 2004), skin (Breuninger et al, 1997) and prostate cancer
(Yanagisawa et al, 2007) have quantified the cellular components of primary tumours and demonstrated that the tumour composition is associated with patient survival. In CRC, the number of
stromal myofibroblasts (Tsujino et al, 2007), vimentin expression (Ngan et al, 2007) and degree of stromal desmoplasia (Halvorsen and Seim, 1989; Shepherd et al, 1997; Sis et al, 2005) have
been associated with patient prognosis in the past. So far, only one study has suggested that the proportion of tumour cells in CRC may be important (Mesker et al, 2007). However, this
result was based upon a relatively small number of cases using qualitative visual estimation of the epithelial component rather than measuring the components objectively by a
well-established morphometric method, such as point counting that was first described in the 1940s (Chalkley, 1943) and developed further by Weibel in the 1960s and 70s (Weibel, 1969). We
hypothesised that the relative proportion of tumour (PoT) determined by objective point counting on virtual (scanned) haematoxylin and eosin-stained slides is related to cancer-specific
survival in CRC patients. MATERIALS AND METHODS PATIENTS A total of 145 patients who had potentially curative resections for colorectal adenocarcinoma at the Marienhospital, Düsseldorf,
Germany between January 1990 and December 1995 were selected for this study. None of the patients had received pre-operative chemotherapy or radiotherapy. The median follow-up time was 4.3
years (interquartile range=2.2–6.2 years) and 107 patients (73.8%) were alive at the end of the study period. CLINICOPATHOLOGICAL DATA Histopathological staging data was obtained from the
pathology reports or from slide review by one of the pathologists (WM) and included the site of the tumour, extramural vascular invasion status, maximum depth of invasion (pT), lymph node
involvement (pN) and distant metastasis (pM) according to TNM classification, 5th edition (Sobin and Wittekind, 1997, pp 66–69). In addition, we had access to data regarding patient age at
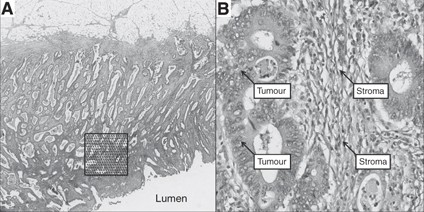
diagnosis and whether or not adjuvant therapy was given. MEASUREMENT OF THE RELATIVE POT Four μm thick haematoxylin and eosin-stained tissue sections prepared according to standard protocols
from one tumour block representing the deepest tumour infiltration into the wall and the largest tumour volume were scanned at × 40 magnification with an automated scanning system (Aperio
XT, Aperio Technologies, Vista, CA, USA). Using a digital slide viewer (ImageScope v8.0, Aperio Technologies), slides were inspected after scanning and a number of preliminary analyses were
carried out on a small number of cases to establish the optimal frequency of points and optimal size of the area required to accurately assess the PoT (data not shown). An area of 9 mm2 was
selected from the luminal surface of each case in which the tumour cell density seemed to be greatest after visual inspection. A grid with a systematic random sample of 300 points was then
superimposed on the selected area using newly developed virtual graticule software (RandomSpot, University of Leeds, Leeds, UK) to count the number of times the point fell over each of the
categories. Large areas of necrosis and mucus at the surface were avoided when selecting the area. The number of necessary measurement points established in our preliminary work was
consistent with previous descriptions of the number of points needed to accurately assess PoT (Baak et al, 1991, pp 189–209). The following categories were used in the scoring system: tumour
(the point falls onto a viable cancer cell), stroma, tumour lumen, necrosis, vessel, inflammation and non-informative (unclassifiable). One of the authors (MD) was trained by an experienced
pathologist (HG) in recognising the different categories and subsequently navigated through each point and categorised the material underneath the point while blinded to the
histopathological and survival data (Figure 1). To assess interobserver variation, a random sample of 40 cases were double scored by a second pathologist (GH). As MD and GH agreed in 98.3%
of the counts (_κ_=0.971), double scoring of all counts was felt to be unnecessary. The PoT was expressed as a percentage fraction of all the informative points per case. Each case took
approximately 20 min to score. STATISTICAL ANALYSES Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS v15.0, Chicago, IL, USA). Comparisons
between PoT and clinicopathological variables were performed using the Mann–Whitney _U_ or Kruskal–Wallis test as appropriate. Correlation analyses were performed using Pearson's
correlation coefficients. The primary endpoint was death attributable to CRC. Cancer-specific survival data were available for all patients. Using a modified receiver operating
characteristic curve approach developed by one of the authors (PMcS), the cut off for dichotomisation of PoT with the highest sensitivity and specificity regarding survival prediction was
calculated (Figure 2). Using this approach, PoT was classified as either PoT-high (>47% of tumour cells within the tumour) or PoT-low (⩽47% of tumour cells within the tumour). Patients
who died within 30 days after surgery (post-operative mortality) were excluded from the study. Univariate survival analyses were performed using Kaplan–Meier curves (Kaplan and Meier, 1958)
and differences between the groups assessed with the log-rank test. To assess whether the potential new prognostic marker predicts survival independent from known prognostic markers, such as
pT, pN, extramural blood vessel invasion and age, multivariate survival analyses were performed using the Cox proportional hazards regression model (Cox, 1972). _P-_values of less than 0.05
were considered to be statistically significant. ETHICAL APPROVAL Ethical approval for the study was granted by the Northern and Yorkshire Research Ethics Committee, Jarrow, UK (unique
reference number 08/H0903/62). RESULTS CLINICOPATHOLOGICAL DATA The median age of patients was 69 years (interquartile range from 61 to 76 years). The remaining clinicopathological data is
displayed in Table 1. RELATIVE PROPORTION OF TUMOUR (POT) The PoT value followed a normal distribution across the series ranging from 21.6 to 84.3% (see Figure 3). The median PoT value was
57.1% (interquartile range from 47.4 to 66.4%). The relationship between high and low PoT with clinicopathological data is shown in Table 1. There was no significant correlation between PoT
and any of the clinicopathological variables, however, a low PoT was weakly related to pT stage rising from 9% in pT1 to 42% in pT4. The frequency of low PoT was higher in cancers of the
rectum compared with cancers of the colon, however, this was not statistically significant (33 _vs_ 21%, _P_=0.125). The PoT value was strongly inversely correlated with the proportion of
stroma within the tumour (_r_=−0.913, _P_<0.0001). SURVIVAL ANALYSES The results of the survival analyses for all cases are shown in Table 2. On univariate analysis, PoT-low was
associated with poorer cancer-specific survival (hazard ratio (HR)=2.087, 95% confidence interval (CI)=1.088–4.003, _P_=0.024, Figure 4). This was also significant when follow-up was
censored at 3 years (HR=2.878, 95% CI=1.369–6.049, _P_=0.003) and 5 years (HR=2.083, 95% CI=1.066–4.072, _P_=0.028). Multivariate analysis confirmed that PoT-low was an independent poor
prognostic marker when the model was adjusted for age, pT stage, pN stage and extramural vascular invasion (_P_=0.017). Subgroup analyses by tumour location demonstrated that PoT is a
significant prognostic factor in colonic cancers (HR=2.474, 95% CI=1.132–5.408, _P_=0.019) but not in rectal cancers (HR=1.693, 95% CI=0.516–5.562, _P_=0.380). This result was confirmed on
multivariate analysis for both colonic (HR=2.703, 95% CI=1.183–6.175, _P_=0.018) and rectal cancers (HR=1.610, 95% CI=0.471–5.505, _P_=0.448). Table 3 shows the survival analyses according
to the TNM stage of disease. The prognostic effect of PoT remained in TNM stage III disease on both univariate (HR=3.480, 95% CI=1.325–9.136, _P_=0.007) and multivariate analysis (HR=3.121,
95% CI=1.091–8.929, _P_=0.034). DISCUSSION Malignant tumours such as CRC are supported by a rich network of stroma that undergoes varying degrees of modification after epithelial cell
invasion including desmoplasia (Hewitt et al, 1993), angiogenesis and inflammatory cell infiltration (De Wever and Mareel, 2003). The composition of tumours varies dramatically among
patients and has previously been linked to differential survival in the breast (Baak et al, 1985), lung (Nakajima, 1991; Maeshima et al, 2002; das Neves Pereira et al, 2004), skin
(Breuninger et al, 1997), prostate cancer (Yanagisawa et al, 2007) and CRC (Halvorsen and Seim, 1989; Shepherd et al, 1997; Sis et al, 2005; Ngan et al, 2007; Tsujino et al, 2007). Most of
these studies demonstrate that a greater proportion of stroma or an exaggerated desmoplastic response is associated with poorer patient outcomes (Jass, 1986; Roncucci et al, 1996). Studies
in human colon cancer cell lines have shown that organ-specific fibroblasts can directly influence the ability of the tumour cells to invade through the production of factors such as
collagenases (Fabra et al, 1992). Molecular studies have shown that specific chromosomal aberrations are related to the proportion of tumour in CRC and therefore may affect tumour–stroma
interactions (Fijneman et al, 2007). Quantitative methods to evaluate desmoplasia including computer-assisted image analysis have already been shown to improve reproducibility of the
assessment and have confirmed a relationship to survival (Sis et al, 2005; Tsujino et al, 2007). Not surprisingly, our study demonstrated that the proportion of viable tumour cells within a
tumour is strongly correlated to the proportion of stroma. Using quantitative point counting on virtual tissue sections, we have shown that a low proportion of malignant epithelial cells
within a given CRC is independently associated with worse cancer-specific survival. The prognostic effect of PoT seemed to be more significant in colonic cancers and TNM stage III disease
than rectal cancers and TNM stages I and II, although due to the small numbers involved in these subgroup analyses the results must be viewed with caution and need to be confirmed in a
larger series of cases. There are several theories as to why a low PoT (and therefore high proportion of stroma) within a tumour may infer a poor prognosis. First, it has been hypothesised
that tumours with a greater proportion of reactive stroma are able to produce more growth factors thus increasing the overall tumour burden (De Wever and Mareel, 2003). Second, it has been
suggested that the relative amount of desmoplastic fibrosis may have a role in reducing the accessibility of tumours to the immune response (Kouniavsky et al, 2002) by encapsulating the
malignant cells and preventing their destruction (Liotta et al, 1983). However, one must consider that PoT may reflect the stage of disease, and our study suggests a weak correlation between
PoT-low and pT stage, which is in concordance with one previous study in which low PoT was seen in 8% of stage I patients and in up to 69% of stage III patients (Mesker et al, 2007).
Morphometrical analysis enables accurate quantification of various tissue components when compared with qualitative systems. The traditional method of using optical graticules on
conventional glass slides has limited flexibility. Using software that can insert any number of sampling points within a systematic grid onto a virtual slide (Treanor et al, 2008), scores
can be entered and saved as an electronic file for further analysis. Although we have used primary resection material in this study, analysis of stromal grade has been shown to be prognostic
on biopsy material from the prostate (Yanagisawa et al, 2007) and our technique could potentially be used on pre-operative biopsies of CRC. We and others have observed significant
heterogeneity in the PoT within individual tumours (Mesker et al, 2007). In this study, we selected an area at the luminal surface to allow future investigation to determine whether the
results may be extrapolated to diagnostic biopsy material. It is possible that different results may be acquired if the measurements were performed in different areas of the tumour, for
example, the centre or the advancing edge. In breast cancer, some studies have shown a better prognosis with higher PoT at the tumour periphery (Baak et al, 1985), whereas others have
suggested an inverse relationship when assessing the whole tumour (Tanaka et al, 2004). Much of the recent research into optimising CRC patient management has focussed on identifying (i)
prognostic markers that allow us to determine which patients may benefit from adjuvant therapy and (ii) predictive markers which predict the response of individual patients to specific
therapeutic regimens. The identification of patients with a poor prognosis based on an estimate of PoT obtained through simple, relatively inexpensive morphometrical measurements could
easily be transferred into routine diagnostic practice according to our own experience. On the basis of the results of our present study, we hypothesise that patients with PoT-low may be
more likely to respond to agents directed at inhibiting the crosstalk between stromal and epithelial cells, an area that clearly warrants further investigations. CHANGE HISTORY * _ 16
NOVEMBER 2011 This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Baak JPA, Van Dop H, Kurver PHJ,
Hermans J (1985) The value of morphometry to classic prognosticators in breast cancer. _Cancer_ 56: 374–382 Article CAS Google Scholar * Baak JPA, Langley FA, Hermans J (1991)
Classification and prognosis for new cases: some aspects of univariate and multivariate analysis. In _Manual of Quantitative Pathology in Cancer Diagnosis and Prognosis_ Baak JPA (ed), pp
189–209. Springer-Verlag: Berlin Google Scholar * Breuninger H, Schaumburg-Lever G, Holzschuh J, Horny HP (1997) Desmoplastic squamous cell carcinoma of skin and vermilion surface. A highly
malignant subtype of skin cancer. _Cancer_ 79: 915–919 Article CAS Google Scholar * Cancer Research UK (2009) (Accessed March 2009) Cancer Stats.
http://info.cancerresearchuk.org/cancerstats/types/bowel/ * Chalkley HW (1943) Methods for quantitative morphological analysis of tissue. _J Natl Cancer Inst_ 4: 47–53 Google Scholar * Cox
DR (1972) Regression models and life tables. _J Royal Stat Soc Series B_ 34: 187–220 Google Scholar * Das Neves Pereira JC, da Silva AG, Soares F, Ab′Saber AM, Schmidt A, Rodrigues OR,
Garippo A, Capelozzi M, de Campos JR, Takagaki T, Jatene FB, Martins S, Capelozzi VL (2004) Nuclear and environment morphometric profile in tumor size and nodal metastasis of resected
typical pulmonary carcinoid. _Pathol Res Pract_ 200: 459–467 Article Google Scholar * De Wever O, Mareel M (2003) Role of tissue stroma in cancer cell invasion. _J Pathol_ 200: 429–447
Article CAS Google Scholar * Fabra A, Nakajima M, Bucana CD, Fidler IJ (1992) Modulation of the invasive phenotype of human colon carcinoma cells by organ-specific fibroblasts of nude
mice. _Differentiation_ 52: 101–110 Article CAS Google Scholar * Fijneman RJ, Carvalho B, Postma C, Mongera S, van Hinsbergh VW, Meijer GA (2007) Loss of 1p36, gain of 8q24, and loss of
9q34 are associated with stroma percentage of colorectal cancer. _Cancer Lett_ 258: 223–229 Article CAS Google Scholar * Gabriel WB, Dukes C, Bussey HJR (1935) Lymphatic spread in cancer
of the rectum. _Br J Surgery_ 23: 395–413 Article Google Scholar * Halvorsen TB, Seim E (1989) Association between invasiveness, inflammatory reaction, desmoplasia and survival in
colorectal cancer. _J Clin Pathol_ 42: 162–166 Article CAS Google Scholar * Hewitt RE, Powe DG, Carter I, Turner DR (1993) Desmoplasia and its relevance to colorectal tumour invasion.
_Int J Cancer_ 53: 62–69 Article CAS Google Scholar * Jass J (1986) Lymphocytic infiltration and survival in rectal cancer. _J Clin Pathol_ 39: 585–589 Article CAS Google Scholar *
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. _J Am Stat Assoc_ 53: 457–481 Article Google Scholar * Kouniavsky G, Khaikin M, Zvibel I, Zippel D, Brill
S, Halpern Z, Papa M (2002) Stromal extracellular matrix reduces chemotherapy-induced apoptosis in colon cancer cell lines. _Clin Exp Metastasis_ 19: 55–60 Article CAS Google Scholar *
Liotta LA, Rao CN, Barsky SH (1983) Tumor invasion and the extracellular matrix. _Lab Invest_ 49: 636–649 CAS PubMed Google Scholar * Maeshima AM, Niki T, Maeshima A, Yamada T, Kondo H,
Matsuno Y (2002) Modified scar grade. A prognostic indicator in small peripheral lung adenocarcinoma. _Cancer_ 95: 2546–2554 Article Google Scholar * Mesker WE, Junggeburt JMC, Szuhai K,
de Heer P, Morreau H, Tanke HJ, Tollenaar RAEM (2007) The carcinoma–stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumour stage.
_Cell Oncol_ 29: 387–398 PubMed PubMed Central Google Scholar * Morris EJ, Maughan NJ, Forman D, Quirke P (2007) Who to treat with adjuvant therapy in Dukes B/stage II colorectal cancer?
The need for high quality pathology. _Gut_ 56: 1419–1425 Article Google Scholar * Nakajima I (1991) Immunohistochemical study of the extracellular matrix in non-small cell lung cancer:
relation to lymph node metastasis and prognosis. _Hokkaido Igaku Zasshi_ 66: 356–368 CAS PubMed Google Scholar * Ngan CY, Yamamoto H, Seshimo I, Tsujino T, Man-i M, Ikeda J-I, Konishi K,
Takemasa I, Ikeda M, Sekimoto M, Matsuura N, Monden M (2007) Quantitative evaluation of vimentin expression in tumour stroma of colorectal cancer. _Br J Cancer_ 96: 986–992 Article CAS
Google Scholar * Roncucci L, Fante R, Losi L, DiGregorio C, Micheli A, Benatti P, Madenis N, Ganazzi D, Cassinadri MT, Lauriola P, Ponz de Leon M (1996) Survival for colon and rectal cancer
in a population based cancer registry. _Eur J Cancer_ 32: 295–302 Article Google Scholar * Shepherd NA, Baxter KJ, Love SB (1997) The prognostic importance of peritoneal involvement in
colonic cancer: a prospective evaluation. _Gastroenterology_ 112: 1096–1102 Article CAS Google Scholar * Sis B, Sarioglu S, Sokmen S, Sakar M, Kupelioglu A, Fuzun M (2005) Desmoplasia
measured by computer assisted image analysis: an independent prognostic marker in colorectal cancer. _J Clin Pathol_ 58: 32–38 Article CAS Google Scholar * Sobin LH, Wittekind Ch (1997)
_TNM Classification of Malignant Tumours_ 5th edn, pp 66–69. Wiley-Liss: New York Google Scholar * Tanaka K, Yamamoto D, Yamada M, Okugawa H (2004) Influence of cellularity in human breast
cancer. _The Breast_ 13: 334–340 Article Google Scholar * Treanor D, Dattani M, Quirke P, Grabsch H (2008) Systematic random sampling with virtual slides: a new software tool for tissue
research. Abstract _J Pathol_ 216 (suppl 1): s43 Google Scholar * Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, Monden M (2007) Stromal
myofibroblasts predict disease recurrence for colorectal cancer. _Clin Cancer Res_ 13: 2082–2090 Article CAS Google Scholar * Weibel ER (1969) Stereological principles for morphometry in
electron microscopic cytology. _Int Rev Cytol_ 26: 235–302 Article CAS Google Scholar * Yanagisawa N, Li R, Rowley D, Liu H, Kadmon D, Miles BJ, Wheeler TM, Ayala GE (2007) Stromogenic
prostatic carcinoma pattern (carcinomas with reactive stromal grade 3) in needle biopsies predicts biochemical recurrence-free survival in patients after radical prostatectomy. _Hum Pathol_
38: 1611–1620 Article CAS Google Scholar Download references ACKNOWLEDGEMENTS NPW and PQ are funded by Yorkshire Cancer Research. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Pathology
and Tumour Biology, Leeds Institute of Molecular Medicine, University of Leeds, St James′s University Hospital, Leeds, LS9 7TF, UK N P West, M Dattani, G Hutchins, J Grabsch, D Treanor, P
Quirke & H Grabsch * Centre for Epidemiology and Biostatistics, Institute of Genetics, Health and Therapeutics, University of Leeds, Leeds, LS2 9JT, UK P McShane * Gemeinschaftspraxis
Pathologie, Starnberg, Germany W Mueller Authors * N P West View author publications You can also search for this author inPubMed Google Scholar * M Dattani View author publications You can
also search for this author inPubMed Google Scholar * P McShane View author publications You can also search for this author inPubMed Google Scholar * G Hutchins View author publications You
can also search for this author inPubMed Google Scholar * J Grabsch View author publications You can also search for this author inPubMed Google Scholar * W Mueller View author publications
You can also search for this author inPubMed Google Scholar * D Treanor View author publications You can also search for this author inPubMed Google Scholar * P Quirke View author
publications You can also search for this author inPubMed Google Scholar * H Grabsch View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR
Correspondence to H Grabsch. RIGHTS AND PERMISSIONS From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike
3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE West, N.,
Dattani, M., McShane, P. _et al._ The proportion of tumour cells is an independent predictor for survival in colorectal cancer patients. _Br J Cancer_ 102, 1519–1523 (2010).
https://doi.org/10.1038/sj.bjc.6605674 Download citation * Received: 22 December 2009 * Revised: 25 March 2010 * Accepted: 29 March 2010 * Published: 20 April 2010 * Issue Date: 11 May 2010
* DOI: https://doi.org/10.1038/sj.bjc.6605674 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * colorectal cancer * proportion of tumour * point
counting * virtual slides
