Corneal surface changes in keratoconjunctivitis sicca. Part ii: the mucus component. A non-contact photomicrographic in vivo study in the human cornea
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT _PURPOSE_ Description of mucus on the corneal surface and in the precorneal tear film in keratoconjunctivitis sicca (KCS). _METHODS_ In all, 24 patients with KCS examined with slit
lamp and by non-contact photomicrography. _RESULTS_ Material interpreted as mucus seemed to exist in three varieties: one optically dense, light reflecting, and with definite forms (fine
threads, variously thick and long strands, sheet-like structures); one appearing as small clumps or patches of light-reflecting material adhering to the corneal surface; and one amorphous.
All were present either _per se_, or in various combinations, with or without adherent cell debris. The mucus attached to abnormal (stainable) corneal surface cells. _CONCLUSIONS_ The formed
material strongly resembles formed mucus found in samples from normal conjunctival surface; the light-reflecting clumps or patches adhering to the surface are possibly of the same origin.
The nature of the amorphous material is unclear. Adherence of formed mucus to the corneal surface seems to be related to the presence of abnormal (stainable) surface cells. SIMILAR CONTENT
BEING VIEWED BY OTHERS AN IN VIVO CONFOCAL MICROSCOPY STUDY OF CORNEAL CHANGES IN PATIENTS WITH SYSTEMIC SCLEROSIS Article Open access 27 May 2021 KERATOCONUS Article 24 October 2024 CORNEAL
SCHEIMPFLUG TOPOGRAPHY VALUES TO DISTINGUISH BETWEEN NORMAL EYES, OCULAR ALLERGY, AND KERATOCONUS IN CHILDREN Article Open access 20 December 2021 INTRODUCTION The corneal surface is
covered by a tear film serving clear vision, surface protection, and its nutrition. One of the tear film components is mucus, a group of various mucins produced by the lacrimal glands,
ocular surface epithelium, and conjunctival goblet cells. As found by laser interferometry, the precorneal tear film is composed largely of mucus.1 The precorneal tear film mucus has been
proposed to exist in the form of hydrated gel bound to membrane-bound mucins on the superficial corneal epithelium (glycocalyx); this form of mucus is presumed to serve optical clarity and
ocular comfort.2 Mucus showing definite morphological features has been reported on the conjunctival surface. Light microscopy, with the aid of India ink particles and filter impressions,3
and scanning electron microscopy in conjunctival biopsies,4 have shown that the normal conjunctival surface is covered by mucus in the form of strands, sheets, and granules. This form of
mucus has been suggested to serve the elimination of exfoliated cells and extraneous particles adhering to the surface, by entrapping and removing them during blinks, and by subsequently
transporting them to the fornices in which they appear as ‘mucous thread’. This thread has been found to consist of variously thick fibres arranged in bundles and to contain various cells
and amorphous material, and suggested to function as conveyor band transporting material to be removed towards the inner canthus where it is ultimately expelled.5 In healthy eyes, mucus is
translucent and cannot be discerned with the slit lamp. With alcain blue staining mucus, small amounts of fine punctuate deposits or a very thin flat coating localised peripherally has been
found in one-fifth of normal corneae.6 Excess of mucus occurs in irritative states such as infections, foreign bodies, and KCS. In KCS, alcain blue stained punctuate and membranous mucus
deposits have been found to extend across the lower part of the cornea, and further on to the bulbar conjunctiva.6 The aim of the present study was to investigate _in vivo_ morphology of
(pre) corneal surface mucus in patients with KCS because of _bona fide_ aqueous tear deficiency (Part I). PATIENTS AND METHODS In all, 24 of the patients presented in Part I, Table 1 (nos.
4–27, 23 women, one man, mean age 56.9 years, range 34–76 years) were examined by the same methods as in Part I (slit-lamp observations, non-contact photomicrography, staining with
fluorescein sodium 1%, and rose bengal 1%). RESULTS BEFORE STAINING With the slit lamp, the precorneal tear film showed floating particles. In some corneae, light-reflecting structures
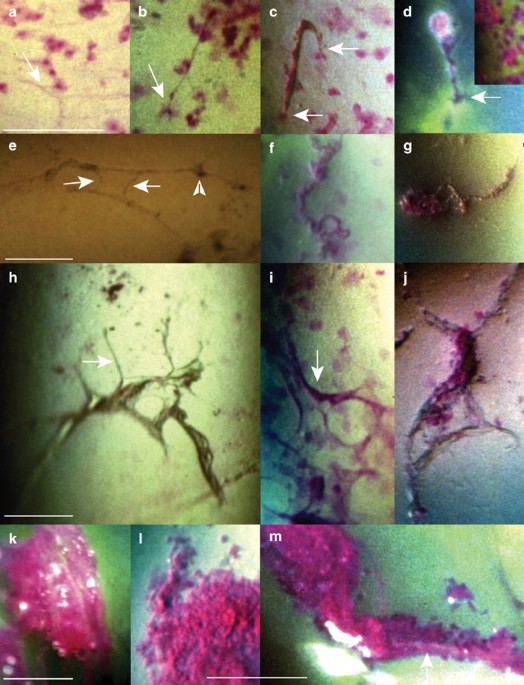
seemed to adhere to the surface. Light-reflecting structures adhering to the surface were captured in the photographs (not shown). AFTER STAINING * Optically dense material with definite
forms: * ∘ Fine threads, apparently lying free on the surface (Figure 1a) or attached at one (Figure 1b and d) or both (Figure 1c) ends to rose bengal stained cells _in situ_ overlying green
stained flecks (Part I) (Figure 1d); rose bengal stained cells/cell debris were adhering to them (Figure 1c and d). Whether or not the fine threads reflected light and stained with rose
bengal was beyond resolution. * ∘ Variously thick and long light-reflecting strands, without (Figure 1e) or with (Figure 1g) attached debris. Some strands were stretched and seemed to be
sticking to the surface at several points (Figure 1e), others formed complex intertwined figures or meshworks (not shown), and some seemed to be lying free on the surface (Figure 1f and g).
Occasionally, some of the attached strands suddenly changed appearance after a blink. Some strands stained red with rose bengal (Figure 1f), others appeared unstained (Figure 1e). * ∘
Sheet-like structures, lying free on the surface (Figure 1h and j) or apparently adhering to it (Figure 1i). Some showed emanating strands and rounded holes (Figure 1h and i), others
appeared folded or collapsed (Figure 1j). They seemed to be light reflecting; some stained with rose bengal (Figure 1i), others did not (Figure 1h and j). * Small patches or small clumps of
light-reflecting material, adhering to the surface (Figure 1d, inset) and staining brightly with rose bengal. * Amorphous material staining red with rose bengal (Figure 1k), sometimes
containing cells debris (Figure 1m) and air bubbles. It was adhering to or enveloped by folded sheet-like structures (Figure 1j), adhering to the strands (Figure 1m) or apparently lying free
on the surface (Figure 1k). * Additional findings were rose bengal stained clumps, apparently lying free on the surface and containing various amounts of cell debris (Figure 1l), and
complex figures consisting of rose bengal stained or unstained strands, light-reflecting material and cell debris; they were attached at several points to areas of green flecks located close
to each other (shown elsewhere7). DISCUSSION In the interpretation of the findings, two possible sources of artefacts had to be considered: topical treatment and the staining with
fluorescein sodium and rose bengal dyes. Topical treatment did not seem to be a factor in the patients who either did not use any, or were examined at least 1 h after the last drop of a tear
substitute. Fluorescein sodium has no known toxic properties.8 The propensity of the light-reflecting material to adhere to the corneal surface was visible before staining with rose bengal,
and thus seemed to be unrelated to the toxic effect on the epithelium of the dye.9 Lid squeezing because of the smarting property of rose bengal dye probably resulted in mechanical
displacements. Material interpreted as mucus comprised three morphological variations, one with definite forms (threads, strands, sheets), one adhering to surface in small clumps or patches,
and one amorphous. The additional material involved was cell debris. The formed material showed striking similarities to formed mucus present on normal conjunctival epithelial surface
(clusters, granular sheets and strands,3 and fine and coarser mucus strands, sheets and granules4). The mechanisms behind the development of strands and sheets on the normal conjunctival
surface are not fully understood. It has been suggested that the strands are the result of sheets rolling up at their edges,3 or that the sheets are the result of the flattening of thicker
strands by the upper eyelid.4 _In vivo_, the single- or double-attached minifilaments seemed to be the result of threads and strands rolling between the tarsal and corneal surfaces,
detaching from the conjunctiva, at random attaching to anchored abnormal (stainable) corneal surface cells, and attaching to themselves exfoliating cells and cell debris. The bonds to
anchored cells seemed to be strong enough to withstand, at least for some time, the shearing action of the lids. When captured in appropriate illumination, areas around their attachments
showed green fluorescein staining indicating disruptions in the epithelial barrier. This might have been due either to mechanical forces (such as tugging on the epithelium during blinks), or
to attachments to abnormal cells present in preformed areas of green flecks (Part I). Also, the small patches and clumps of optically dense material (corresponding to clusters or granulae
on the normal conjunctiva?3,4), as well as some of the sheet-like structures apparently detached in toto from the (superior tarsal?) conjunctiva, and more complex structures resembling mucus
plaques reported previously in KCS10 adhered to abnormal surface cells. The second component captured in the present study, the amorphous material, stained with rose bengal and seemed to
have an adhesive quality, as seen by its propensity to adhere to or envelop the strands, and to cement exfoliated cells/cell debris. Whether or not this apparently sticky material also
adhered to abnormal cells _in situ_ and promoted adherences to the surface of the formed material could not be decided by present means. It is unclear whether the amorphous material
represented an excess of mucus, perhaps corresponding to amorphous material found in normal eyes in the ‘mucous thread’,5 or mucus with altered composition. The present 24 patients, 23
(95.8%) of whom had a systemic disease, represented the more severe end of KCS, as estimated by the extent of surface changes revealed with rose bengal dye. In this condition, ‘mucus excess’
is regularly seen with the slit lamp. Examined on a higher magnification level, this mucus appeared to consist partly of formed conjunctival mucus released in the tear film, and partly of
amorphous material the composition of which is unclear. Under normal conditions, formed conjunctival mucus does not adhere to the corneal surface. For unclear reasons, surface adherence
occurs in a variety of disease conditions including KCS. In KCS, the propensity of formed conjunctival mucus to bind to the surface seems to be related to the presence of abnormal
(stainable) surface cells. It is conceivable but not proven that the amorphous material might partly function as a mediator cementing formed mucus to diseased cells, and that its dissolution
might be the mechanism behind the beneficial effect of mucolytic agents on filamentary keratitis11 and corneal mucus plaques.10 REFERENCES * Prydal JI, Artal P, Woon H, Campbell FW . Study
of human precorneal tear film thickness and structure using laser interferometry. _Invest Ophthalmol Vis Sci_ 1992; 33: 2006–2011. CAS PubMed Google Scholar * Pflugfelder SC, Solomon A,
Stern ME . The diagnosis and management of dry eye. A twenty-five-year review. _Cornea_ 2000; 19: 644–649. Article CAS Google Scholar * Adams AD . The morphology of human conjunctival
mucus. _Arch Ophthalmol_ 1979; 97: 730–734. Article CAS Google Scholar * Greiner JV, Korb DR, Covington HI, Peace DG, Allansmith MR . Human ocular mucus. Scanning electron microscopic
study. _Arch Ophthalmol_ 1982; 100: 1614–1617. Article CAS Google Scholar * Egeberg J, Norn MS . Ultrastructure of mucous thread in inferior conjunctival fornix. _Acta Ophthalmol_ 1967;
45: 727–732. Article CAS Google Scholar * Norn MS . Mucus on conjunctiva and cornea. _Acta Ophthalmol_ 1963; 41: 13–24. Article CAS Google Scholar * Tabery HM . Corneal epithelial
keratitis in herpes zoster ophthalmicus: ‘delayed’ and ‘sine herpete’. A non-contact photomicrographic _in vivo_ study in the human cornea. _Eur J Ophthalmol_ 2002; 12: 267–275. Article CAS
Google Scholar * Feenstra RPG, Tseng SCG . Comparison of fluorescein and rose bengal staining. _Ophthalmology_ 1992; 99: 605–617. Article CAS Google Scholar * Tabery HM . Toxic effect
of rose bengal dye on the living human corneal epithelium. _Acta Ophthalmol Scand_ 1998; 76: 142–145. Article CAS Google Scholar * Fraunfelder FT, Wright P, Tripathi RC . Corneal mucus
plaques. _Am J Ophthalmol_ 1977; 83: 191–197. Article CAS Google Scholar * Jones BR, Coop H . The management of keratoconjunctivitis sicca. _Trans Ophthalmol Soc UK_ 1965; 85: 379–391.
CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by a grant from Herman Järnhardts Stiftelse. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Ophthalmology, Malmö University Hospital, Malmö, Sweden H M Tabery Authors * H M Tabery View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHOR Correspondence to H M Tabery. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tabery, H. Corneal surface changes in
keratoconjunctivitis sicca. Part II: the mucus component. A non-contact photomicrographic _in vivo_ study in the human cornea. _Eye_ 17, 488–491 (2003).
https://doi.org/10.1038/sj.eye.6700400 Download citation * Received: 04 March 2002 * Accepted: 09 November 2002 * Published: 15 May 2003 * Issue Date: 01 May 2003 * DOI:
https://doi.org/10.1038/sj.eye.6700400 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * keratoconjunctivitis sicca * mucus * cornea * human
