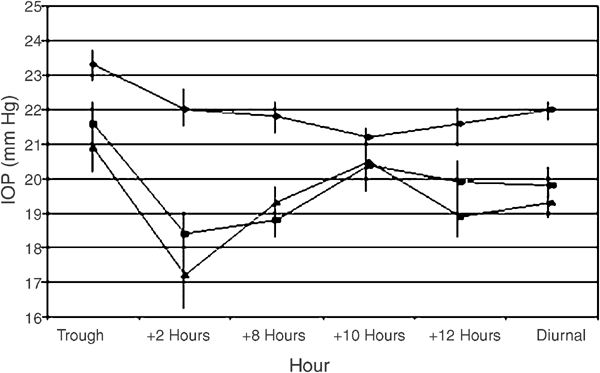
Brimonidine 0.2% vs unoprostone 0.15% both added to timolol maleate 0.5% given twice daily to patients with primary open-angle glaucoma or ocular hypertension
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

To compare the efficacy and safety of brimonidine 0.2% vs unoprostone 0.15%, both added to timolol maleate 0.5% each given twice daily.
In this prospective, multi-centred, double-masked, crossover comparison, patients were randomized to one treatment group for a 6-week treatment period, and then crossed over to the opposite
treatment. Measurements were performed at 0800, 1000, 1600, 1800, and 2000 h at baseline and at the end of each treatment period.
In all, 33 patients entered this trial and 29 completed. The baseline trough intraocular pressure (IOP) was 23.3±2.4 and the diurnal curve IOP was 22.0±1.3 mmHg. For the brimonidine and
timolol maleate treatment group, the trough IOP was 21.6±3.3 and the diurnal curve IOP was 19.8±2.1 mmHg, while the timolol and unoprostone treatment showed a trough IOP of 20.9±3.8 and a
diurnal curve IOP of 19.3±2.4 mmHg. There was no significant difference between treatment groups at any time point for the diurnal curve, or in the reduction from baseline (P>0.05). Both
treatments failed to statistically reduce the IOP from baseline at 1800 h. There was no difference between treatment groups regarding ocular and systemic unsolicited adverse events, but
patients admitted to more dryness (P=0.02) and burning upon instillation (P
