Branch retinal vein occlusion treated by intravitreal triamcinolone acetonide
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT _PURPOSE_ To evaluate the effect of intravitreal triamcinolone acetonide on visual acuity in branch retinal vein occlusion. _METHODS_ The prospective comparative nonrandomized
clinical interventional study included 28 patients (28 eyes) with branch retinal vein occlusion. The study group consisting of 10 consecutive patients received an intravitreal injection of
20–25 mg of triamcinolone acetonide. The control group including 18 patients did not receive an intravitreal injection. The mean follow-up was 8.7±4.4 months. _RESULTS_ In the study group,
mean visual acuity increased significantly (_P_=0.02) from 0.27±0.11 preoperatively to a best postoperative visual acuity of 0.45±0.27. Visual acuity measurements determined 1 month after
the injection were significantly (_P_=0.027) higher than baseline values. Nine (90%) eyes gained in visual acuity, with six (60%) eyes showing an increase in visual acuity of at least two
Snellen lines. In the ischaemic subgroup, visual acuity did not change significantly (0.18±0.18 to 0.13±0.04; _P_=0.66), while, in the nonischaemic subgroup, visual acuity increased
significantly (_P_=0.012) from the baseline value to the best postoperative measurement (0.29±0.09 to 0.53±0.24). In the control group, baseline visual acuity and best visual acuity during
the follow-up did not vary significantly (_P_=0.27). Comparing the study and control groups with each other, the gain in visual acuity was significantly higher in the study group at 1 month
(_P_=0.016) and 2 months (_P_=0.012) after baseline. _CONCLUSIONS_ Intravitreal injection of triamcinolone acetonide can increase visual acuity in patients with branch retinal vein
occlusion. SIMILAR CONTENT BEING VIEWED BY OTHERS EFFICACY OF AS-NEEDED INTRAVITREAL INJECTION COMPARED TO 3-MONTHLY LOADING OF ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR AGENTS FOR BRANCH
RETINAL VEIN OCCLUSION Article Open access 26 July 2023 CENTRAL RETINAL ARTERY CATHETERIZATION FOR RETINAL ARTERY OCCLUSION WITH BALANCED SALT SOLUTION Article Open access 02 April 2025
CHARACTERISTICS OF MAJOR AND MACULAR BRANCH RETINAL VEIN OCCLUSION Article Open access 18 August 2022 INTRODUCTION Intravitreal triamcinolone acetonide has increasingly been used in previous
studies for treatment of intraocular proliferative, oedematous, and neovascular diseases, such as diffuse diabetic macular oedema,1, 2, 3 proliferative diabetic retinopathy,4 neovascular
glaucoma,5 central retinal vein occlusion,6, 7, 8 proliferative vitreoretinopathy,9 chronic pre-phthisical ocular hypotony,10 chronic uveitis,11, 12, 13, 14, 15 persistent pseudophakic
cystoid macular oedema,16, 17, 18 exudative age-related macular degeneration,19, 20, 21, 22, 23, 24, 25 cystoid macular oedema due to retinitis pigmentosa,26 ischaemic ophthalmopathy,27
sympathetic ophthalmia,28 idiopathic juxtafoveal telangiectasis,29 as visualization aid during pars plana vitrectomy,30 and in other clinical situations.31, 32, 33, 34, 35, 36 In aqueous
humour and in silicone oil, respectively, triamcinolone acetonide has been found up to 1.5 years and up to 8 months, respectively, after the intravitreal injection.37, 38, 39, 40 Systemic
and local side effects reported so far include cataract, secondary ocular hypertension, leading in some patients to secondary chronic open-angle glaucoma,41, 42, 43 and postinjection
infectious endophthalmitis.44, 45, 46 Due to its antioedematous and antiangiogenic effects as shown in experimental investigations and clinical studies,47, 48, 49, 50, 51 intravitreal
triamcinolone acetonide has additionally been used in previous pilot studies on central retinal vein occlusions. It was the purpose of the present study to evaluate whether intravitreal
triamcinolone acetonide may be therapeutically useful to increase visual acuity in patients with long-standing cystoid macular oedema due to branch retinal vein occlusion. METHODS The
prospective comparative nonrandomized clinical interventional study included all 10 patients (five women; 10 eyes; six right eyes), who consecutively presented with branch retinal vein
occlusion, who received an intravitreal injection of 20–25 mg of triamcinolone acetonide, who did not undergo cataract surgery in combination with, or after, the intravitreal injection, and
for whom follow-up was 1 month or longer (Table 1). All patients were fully informed about the experimental character of the therapy. All patients signed an informed consent. The study was
performed at a university hospital. The ethics committee of the university had approved the study following the tenets of the Declaration of Helsinki. The mean age of the patients was
73.9±7.3 years, mean refractive error was 1.01±1.70 D (Table 1). Mean follow-up time was 8.7±4.4 months. Fluorescein angiogram performed for all patients showed a marked cystoid macular
oedema, in addition to intraretinal haemorrhages in the region of the branch retinal vein occlusion. Two eyes (20%) showed the ischaemic type of branch retinal vein occlusion, and the
remaining eight (80%) eyes showed the nonischaemic type of branch retinal vein occlusion. The study group was compared with a control group including 18 eyes (nine right eyes) of 18 patients
(10 women), who did not receive an intravitreal injection of triamcinolone acetonide. In four (22%) eyes, a retinal argon laser coagulation was performed as treatment of branch retinal vein
occlusion during the follow-up. Five (28%) patients of the control group showed the ischaemic type of branch retinal vein occlusion, and 13 (72%) showed the nonischaemic type. Due to the
distribution of patients into the study group and the control group, both groups did not vary significantly in preoperative visual acuity (_P_=0.27), preoperative intraocular pressure
(_P_=0.19), age (_P_=0.11), refractive error (_P_=0.46), gender (_P_=1.01), right or left eye (_P_=0.71), follow-up time (_P_=0.13), and ischaemic _vs_ nonischaemic type of branch retinal
vein occlusion (_P_=1.0) (Table 1). All patients complained about a loss of vision experienced at least 5 months prior to the intravitreal injection. The mean visual acuity at baseline of
the study was 0.27±0.11 (median 0.25; range 0.05–0.50) in the study group, and it was 0.35±0.16 (median 0.40; range 0.06–0.50) in the control group. The mean intraocular pressure measured
16.9±3.2 mmHg for the patients of the study group, and it was 15.2±2.9 mmHg for the patients of the control group (Table 1). At baseline of the study and in repeated intervals afterwards,
all patients underwent a routine ophthalmologic examination including standardized visual acuity measurement using Snellen charts, slit-lamp biomicroscopy, Goldmann applanation tonometry,
and ophthalmoscopy. For the patients of the study group, the examinations were routinely performed during the first week after the injection, and roughly in monthly intervals after the
injection. For all patients included in the study, the results of at least one examination performed at least 1 month, or later, after the intravitreal injection were available. The
intravitreal injection of triamcinolone acetonide was performed under sterile conditions in the operation theatre, using an operation microscope. Prior to the intravitreal injection, topical
BetadineR (povidone-iodine 5%) (Alcon, Ft Worth, TX, USA) was applied, and after that the patients were completely draped. A lid speculum was inserted and a paracentesis carried out to
decrease the volume of the eye. The injection of 20–25 mg (0.2 ml) crystalline triamcinolone acetonide was performed through a sharp 27-gauge needle through the inferior pars plana, at 3–3.5
mm from the limbus. After that, an antibiotic ointment (polymyxin and neomycine) was applied. The triamcinolone acetonide had been prepared by extracting 0.625 ml from the ampoule (Volon
AR, Bristol-Myers-Squibb, Germany) containing 40 mg of triamcinolone acetonide in 1 ml. The extracted volume was filled into a tuberculin syringe (1 ml) or a 2 ml syringe. The syringe was
filled up with Ringer's solution. A millipore filter (pore size, 5 _μ_m) was placed on top of the syringe, and most of the content of the syringe was pressed through the filter, with
the triamcinolone acetonide crystals remaining in the syringe. The syringe was re-filled with Ringer's solution, and the same procedure was repeated for three times. At the end, 0.2 ml
of solution in the syringe was left, and, using a 27-gauge needle, the content was injected transconjunctivally into the vitreous cavity. Statistical analyses were performed by using a
commercially available statistical software package (SPSS for Windows, version 11.5, SPSS, Chicago, IL, USA). To test the statistical significance of differences between the study group and
the control group, the Mann–Whitney test, Wilcoxon–test, or Student's-_t_-test for parameters such as intraocular pressure and visual acuity were used. For parameters such as gender and
right or left eye, the _χ_2 test was applied. The level of significance was 0.05 (two-sided) in all statistical testing. RESULTS In the study group, mean visual acuity increased from 0.
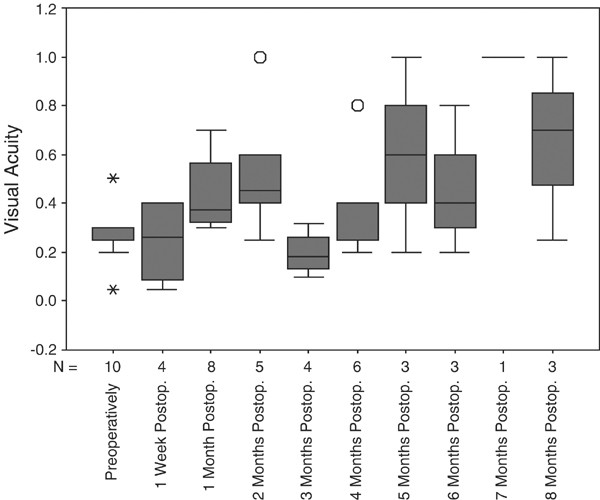
27±0.11 preoperatively to a best visual acuity during the follow-up of 0.45±0.27 (Table 2; Figure 1). The difference between the baseline value and the best postoperative value was
statistically significant (_P_=0.02). Nine (90%) eyes showed at least one visual acuity measurement better during the follow-up compared with baseline of the study. Measured in Snellen
lines, six (60%) eyes showed an improvement by at least two Snellen lines or more. Visual acuity measurements determined 1 month after the injection were significantly (_P_=0.027) higher
than the baseline values (Table 2). There was no clear tendency of visual acuity measurements to return to the baseline values at the end of the follow-up (Table 2; Figure 1). Considering
visual acuity measurements taken at the end of the follow-up, visual acuity measurements were higher, however not significantly higher, than the values obtained at the baseline of the study.
The difference to the baseline visual acuity was not significant (_P_=0.59) (Table 2). Dividing the study group into the ischaemic subgroup (_n_=2) and the nonischaemic group (_n_=8)
revealed that preoperative visual acuity was lower, however not statistically lower (_P_=0.71), in the ischaemic subgroup _vs_ the nonischaemic subgroup (0.18 ± 0.18 _vs_ 0.29 ± 0.09). In
the ischaemic subgroup, visual acuity did not change significantly (0.18 ± 0.18 to 0.13 ± 0.04; _P_=0.66), while, in the nonischaemic subgroup, visual acuity increased significantly
(_P_=0.012) from the baseline value to the best postoperative measurement (0.29 ± 0.09 to 0.53 ± 0.24). Expressed in Snellen lines, the postoperative increase in visual acuity was higher,
however not significantly (_P_=0.71) higher, in the nonischaemic subgroup than in the nonischaemic subgroup (2.3 ± 1.7 Snellen lines _vs_ 0.0 ± 4.2 Snellen lines). In the control group,
baseline visual acuity and best visual acuity during the follow-up did not vary significantly (0.35 ± 0.16 _vs_ 0.40 ± 0.24; _P_=0.27) (Table 3). Visual acuity at the end of the follow-up
did not differ significantly (_P_=0.89) from the baseline values (Table 3). Eight eyes (44%) showed at least one visual acuity measurement better during the follow-up compared with baseline
of the study. For seven (39%) eyes, visual acuity measurements after baseline of the study were worser than those at the start of the study. Comparing the study and control groups with each
other, gain in visual acuity measured 1 month after baseline of the study was significantly higher in the study group than in the control group (2.30 ± 1.86 Snellen lines _vs_ −1.75± 1.26;
_P_=0.016). The same hold true for visual acuity measurements obtained at 2 months after the start of the study (_P_=0.012). For visual acuity measurements performed later during the
follow-up, the number of patients may have been too small for a statistical analysis (Tables 2 and 3). Maximal gain in visual acuity was higher, however not significantly (_P_=0.12) higher,
in the study group (1.89 ± 2.26 Snellen lines) than in the control group (0.25 ± 3.34 Snellen lines). The number of eyes demonstrating an improvement in visual acuity during the follow-up
was higher, however not statistically significantly (_P_=0.065), in the study group than in the control group. In the study group, intraocular pressure increased significantly (_P_=0.007)
from 16.9 ± 3.2 mmHg (median 17 mmHg; range 12–23 mmHg) at baseline of the study to a mean maximal value of 23.5 ± 4.4 mmHg (median 23 mmHg; range 18 – 32 mmHg), and decreased significantly
(_P_=0.03) again to 19.8 ± 6.2 mmHg (median 17 mmHg; range 15 – 32 mmHg) at the end of the follow-up. The preoperative intraocular pressure measurements and the pressure readings at the end
of the follow-up period did not differ significantly (_P_=0.27). During the study period, intraocular pressure was higher than 21 mmHg in seven (70%) eyes. In all of these eyes, intraocular
pressure could be normalized by topical antiglaucomatous medication. Glaucomatous damage of the optic nerve as determined by biomorphometry of the optic nerve head12 was not detected. In the
control group, intraocular pressure at baseline of the study (15.2 ± 2.9 mmHg), intraocular pressure measurements during the follow-up (peak: 14.8 ± 4.1 mmHg), and at the end of the study
did not vary significantly (_P_>0.20). None of the eyes of the control group developed intraocular pressure measurements higher than 23 mmHg during the follow-up. Correspondingly,
intraocular pressure during the follow-up (_P_<0.001) and at the end of the study (_P_=0.002) was significantly higher in the study group than in the control group. Both groups did not
vary significantly (_P_=0.19) in intraocular pressure at baseline of the study (Table 1). In none of the patients, triamcinolone acetonide crystals had settled on the macular region. The
crystals were preretinally located in the vitreous cortex at the 6 o’clock position and did not optically interfere with vision. In none of the eyes included in the study, postoperative
infectious endophthalmitis, sterile endophthalmitis, rhegmatogenous retinal detachment or proliferative vitreoretinopathy were observed. DISCUSSION The results of the study suggest that
intravitreal triamcinolone acetonide may be useful to increase visual acuity in patients with branch retinal vein occlusion. The patients of the study group experienced a significant
increase in visual acuity, while the patients of the control group did not show a significant change in visual acuity during the follow-up (Tables 2 and 3; Figure 1). Comparing the study and
control groups with each other, gain in visual acuity was significantly more marked in the study group for the measurements obtained 1 and 2 months after baseline. It confirms previous
studies in which intravitreal triamcinolone acetonide reduced macular oedema in eyes with different diseases such as pseudophakic cystoid macular oedema, uveitis, exudative age-related
macular degeneration, and diffuse diabetic macular oedema.1, 2, 3, 6, 7, 8, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 29 The present study on branch retinal vein
occlusion is in agreement also with studies on patients with retinal vein occlusions in which intravitreal triamcinolone acetonide led to a marked decrease in macular oedema, and to a slight
increase in visual acuity.6, 7, 8 Previous studies on central retinal vein occlusion and exudative age-related macular degeneration demonstrated an increase in visual acuity or a relative
decrease in the size of the subfoveal neovascularization membrane in the first few months after an intravitreal injection of triamcinolone, and they showed a re-decline in visual acuity
about 3–5 months after the injection.6, 7, 8, 19, 20, 21, 22, 23, 24, 25 In contrast to the latter studies, the present investigation did not show a clear tendency of a decrease in visual
acuity towards the end of the study. It may suggest that, in eyes with branch retinal vein occlusion, intravitreal triamcinolone acetonide may be associated with a longer lasting increase in
visual acuity compared with eyes with central retinal vein occlusion and exudative age-related macular degeneration. The reasons why intravitreal steroids increase vision in patients with
macular oedema have been elusive; however, stabilization of the blood–retinal barrier may play a significant part.48, 52 One of the reasons why visual acuity did not increase more than that
found in the present study may be macular ischaemia and tissue destruction accompanying retinal vein occlusions. It is reflected in the finding that, in the ischaemic subgroup of the study
group, visual acuity did not change significantly (_P_=0.66) after the intravitreal injection, while, in the nonischaemic subgroup, visual acuity increased significantly (_P_=0.012). If
retinal cells have been lost due to capillary nonperfusion, or if intraretinal intercellular structures have been disrupted due to chronic macular oedema and swelling, resolution of the
oedema may only have a limited effect in raising visual function. Direct toxic effects of triamcinolone acetonide on the retina and optic nerve were not observed in the present study nor in
other studies on eyes in which the same dosage of triamcinolone acetonide was injected for various reasons.1, 4, 5, 7, 9, 10, 18, 23, 24, 27, 28, 32, 33, 34, 35, 36, 37, 38, 40, 42
Correspondingly, a recent safety and efficacy study of an intravitreal fluocinolone acetonide sustained delivery device as treatment for cystoid macular oedema in patients with uveitis13 and
other clinical and experimental studies have not shown a toxic effect of intraocular steroids.53, 54, 55, 56 An elevation of intraocular pressure above 21 mmHg occurring in seven eyes (70%)
in the present study was not a major clinical problem. In all these eyes, intraocular pressure could be controlled by topical antiglaucomatous treatment. Similar observations were made by
Wingate41 and colleagues, Martidis2 and colleagues, and Bakri and Beer43 using a dosage of 4 mg of triamcinolone acetonide, as well as in other previous studies using a dosage of 20–25 mg of
triamcinolone acetonide.42 A major difference between the studies on the intravitreal application of triamcinolone acetonide performed by others and the present investigation is the dosage
of triamcinolone intravitreally injected. In all previous studies on intravitreal applications of triamcinolone acetonide for cystoid macular oedema, diabetic macular oedema, and macular
degeneration performed by other researchers, dosages of 4 mg or less of triamcinolone acetonide were used. The reasons why we used a dosage of 20–25 mg of triamcinolone acetonide instead of
a dosage of 4 mg have been that, right from the beginning of the ongoing triamcinolone studies now involving more than 500 patients, we have used the same dosage of 20–25 mg of triamcinolone
acetonide, and that we have not seen side effects so far that may be attributed to that high dosage. It also holds true for repeated intravitreal injections of 20–25 mg of triamcinolone
acetonide.23, 25 The most important limitation of the present study is that it is not a randomized prospective investigation in which the patients were randomly distributed between a study
group and a control group. Yet, nine (90%) eyes gained in visual acuity after the injection of triamcinolone acetonide, with six (60%) eyes gaining in visual acuity by at least two Snellen
lines. Furthermore, one has to consider that the effect of a triamcinolone-induced cataract formation on visual acuity has not been taken into account in the assessment of visual acuity in
the present study. The cataract-associated decrease in visual acuity may have compensated or partially covered an increase in visual acuity due to the effect of triamcinolone acetonide.
Other limitations of the study are the relatively small number of patients included in the study group, and the relatively short follow-up period. In conclusion, the patients included in the
present study showed after an intravitreal injection of 20–25 mg of triamcinolone acetonide a significant increase in visual acuity. Side effects of the intravitreal injection of
triamcinolone acetonide were an elevation in intraocular pressure in seven (70%) eyes, and, probably, an increase in cataract formation. A steroid-induced rise in intraocular pressure could
medically be controlled. In agreement with previous case reports on the effect of intravitreal triamcinolone acetonide on macular oedema due to retinal vein occlusions,6, 7, 8, 57 the
results of the present study on branch retinal vein occlusion suggest that the intravitreal injection of triamcinolone acetonide may be a therapeutic option to temporarily increase visual
acuity in patients with branch retinal vein occlusions. REFERENCES * Jonas JB, Söfker A . Intraocular injection of crystalline cortisone as adjunctive treatment of diabetic macular oedema.
_Am J Ophthalmol_ 2001; 132: 425–427. Article CAS Google Scholar * Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E _et al_. Intravitreal triamcinolone for
refractory diabetic macular oedema. _Ophthalmology_ 2002; 109: 920–927. Article Google Scholar * Jonas JB, Kreissig I, Söfker A, Degenring RF . Intravitreal injection of triamcinolone
acetonide for diabetic macular oedema. _Arch Ophthalmol_ 2003; 121: 57–61. Article CAS Google Scholar * Jonas JB, Hayler JK, Söfker A, Panda-Jonas S . Intravitreal injection of
crystalline cortisone as adjunctive treatment of proliferative diabetic retinopathy. _Am J Ophthalmol_ 2001; 131: 468–471. Article CAS Google Scholar * Jonas JB, Hayler JK, Söfker A,
Panda-Jonas S . Regression of neovascular iris vessels by intravitreal injection of crystalline cortisone. _J Glaucoma_ 2001; 10: 284–287. Article CAS Google Scholar * Greenberg PB,
Martidis A, Rogers AH, Duker JS, Reichel E . Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion. _Br J Ophthalmol_ 2002; 86: 247–248. Article
Google Scholar * Jonas JB, Kreissig I, Degenring RF . Intravitreal triamcinolone acetonide as treatment of macular oedema in central retinal vein occlusion. _Graef Arch Clin Exp Ophthalmol_
2002; 240: 782–783. Article Google Scholar * Park CH, Jaffe GJ, Fekrat S . Intravitreal triamcinolone acetonide in eyes with cystoid macular edema associated with central retinal
veinocclusion. _Am J Ophthalmol_ 2003; 136: 419–425. Article CAS Google Scholar * Jonas JB, Hayler JK, Panda-Jonas S . Intravitreal injection of crystalline cortisone as adjunctive
treatment of proliferative vitreoretinopathy. _Br J Ophthalmol_ 2000; 84: 1064–1067. Article CAS Google Scholar * Jonas JB, Hayler JK, Panda-Jonas S . Intravitreal injection of
crystalline cortisone as treatment of pre-phthisical ocular hypotony. _Graef Arch Clin Exp Ophthalmol_ 2001; 239: 464–465. Article CAS Google Scholar * Antcliff RJ, Spalton DJ, Stanford
MR, Graham EM, ffytche TJ, Marshall J . Intravitreal triamcinolone for uveitic cystoid macular oedema: an optical coherence tomography study. _Ophthalmology_ 2001; 108: 765–772. Article CAS
Google Scholar * Martidis A, Duker JS, Puliafito CA . Intravitreal triamcinolone for refractory cystoid macular oedema secondary to birdshot retinochoroidopathy. _Arch Ophthalmol_ 2001;
119: 1380–1383. CAS Google Scholar * Young S, Larkin G, Branley M, Lightman S . Safety and efficacy of intravitreal triamcinolone for cystoid macular oedema in uveitis. _Clin Exp
Ophthalmol_ 2001; 29: 2–6. Article CAS Google Scholar * Degenring RF, Jonas JB . Intravitreal injection of triamcinolone acetonide as treatment of chronic uveitis. _Br J Ophthalmol_ 2003;
87: 361. Article CAS Google Scholar * Sonoda KH, Enaida H, Ueno A, Nakamura T, Kawano YI, Kubota T _et al_. Pars plana vitrectomy assisted by triamcinolone acetonide for refractory
uveitis: a case series study. _Br J Ophthalmol_ 2003; 87: 1010–1014. Article Google Scholar * Benhamou N, Massin P, Haouchine B, Audren F, Tadayoni R, Gaudric A . Intravitreal
triamcinolone for refractory pseudophakic macular oedema. _Am J Ophthalmol_ 2003; 135: 246–249. Article Google Scholar * Conway MD, Canakis C, Livir-Rallatos C, Peyman GA . Intravitreal
triamcinolone acetonide for refractory chronic pseudophakic cystoid macular oedema. _J Cataract Refract Surg_ 2003; 29: 27–33. Article Google Scholar * Jonas JB, Kreissig I, Degenring RF .
Intravitreal triamcinolone acetonide for pseudophakic cystoid macular oedema. _Am J Ophthalmol_ 2003; 136: 384–386. Article CAS Google Scholar * Penfold P, Gyory J, Hunyor A, Billson FA
. Exudative macular degeneration and intravitreal triamcinolone. A pilot study. _Aust NZ J Ophthalmol_ 1995; 23: 293–298. Article CAS Google Scholar * Danis RP, Ciulla TA, Pratt LM,
Anliker W . Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. _Retina_ 2000; 20: 244–250. Article CAS Google Scholar * Gillies MC, Simpson JM, Luo W,
Penfold P, Hunyor AB, Chua W _et al_. A randomized clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age-related macular degeneration: one-year results.
_Arch Ophthalmol_ 2003; 121: 667–673. Article CAS Google Scholar * Spaide RF, Sorenson J, Maranan L . Combined photodynamic therapy with verteporfin and intravitreal triamcinolone
acetonide for choroidal neovascularization. _Ophthalmology_ 2003; 110: 1517–1525. Article Google Scholar * Jonas JB, Kreissig I, Degenring RF . Repeated intravitreal injections of
triamcinolone acetonide as treatment of progressive exudative age-related macular degeneration. Brief Report. _Graef Arch Clin Exp Ophthalmol_ 2002; 240: 872–873. Article Google Scholar *
Jonas JB, Kreissig I, Hugger P, Sauder G, Panda-Jonas S, Degenring R . Intravitreal triamcinolone acetonide for exudative age-related macular degeneration. _Br J Ophthalmol_ 2003; 87:
462–468. Article CAS Google Scholar * Jonas JB, Akkoyun I, Budde WM, Kreissig I, Degenring RF . Intravitreal re-injection of triamcinolone for exudative age-related macular degeneration.
_Arch Ophthalmol_ 2004, (in print). * Saraiva VS, Sallum JM, Farah ME . Treatment of cystoid macular edema related to retinitis pigmentosa with intravitreal triamcinolone acetonide.
_Ophthalmic Surg Lasers Imaging_ 2003; 34: 398–400. PubMed Google Scholar * Jonas JB, Kreissig I, Degenring RF . Intravitreal triamcinolone as treatment for ischaemic ophthalmopathy. _Eur
J Ophthalmol_ 2003; 13: 575–576. Article CAS Google Scholar * Jonas JB . Intravitreal triamcinolone acetonide for treatment of sympathetic ophthalmia. _Am J Ophthalmol_ 2004, (in print).
* Alldredge CD, Garretson BR . Intravitreal triamcinolone for the treatment of idiopathic juxtafoveal telangiectasis. _Retina_ 2003; 23: 113–116. Article Google Scholar * Peyman GA, Cheema
R, Conway MD, Fang T . Triamcinolone acetonide as an aid to visualization of the vitreous and the posterior hyaloid during pars plana vitrectomy. _Retina_ 2000; 20: 554–555. Article CAS
Google Scholar * Rechtman E, Allen VD, Danis RP, Pratt LM, Harris A, Speicher MA . Intravitreal triamcinolone for choroidal neovascularization in ocular histoplasmosis syndrome. _Am J
Ophthalmol_ 2003; 136: 739–741. Article CAS Google Scholar * Scott IU, Flynn HW, Rosenfeld PJ . Intravitreal triamcinolone acetonide for idiopathic cystoid macular edema. _Am J
Ophthalmol_ 2003; 136: 737–739. Article CAS Google Scholar * Jonas JB, Kreissig I, Degenring RF . Cataract surgery after intravitreal injection of triamcinolone acetonide. _Eye_ 2004, (in
print). * Jonas JB . Intravitreal triamcinolone acetonide as treatment of chronic focal immunologic corneal graft reaction. Brief Report. _Graef Arch Clin Exp Ophthalmol_ 2003; 241:
779–780. Article Google Scholar * Jonas JB . Intravitreal triamcinolone acetonide as treatment for extensive exudative retinal detachment? _Br J Ophthalmol_ 2004, (in print). * Jonas JB,
Kreissig I, Degenring RF . Neovascular glaucoma treated by intravitreal triamcinolone acetonide. _Acta Ophthalmol_ 2003; 81: 540–541. Article Google Scholar * Jonas JB . Concentration of
intravitreally applicated triamcinolone acetonide in aqueous humour. _Br J Ophthalmol_ 2002; 86: 1066. Article Google Scholar * Jonas JB . Concentration of intravitreally injected
triamcinolone acetonide in intraocular silicone oil. _Br J Ophthalmol_ 2002; 86: 1450–1451. Article CAS Google Scholar * Beer PM, Bakri SJ, Singh RJ, Liu W, Peters III GB, Miller M .
Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. _Ophthalmology_ 2003; 110: 681–686. Article Google Scholar * Jonas JB .
Intraocular availability of triamcinolone acetonide after intravitreal injection. _Am J Ophthalmol_ 2004, (in print). * Wingate RJ, Beaumont PE . Intravitreal triamcinolone and elevated
intraocular pressure. _Aust N Z J Ophthalmol_ 1999; 27: 431–432. Article CAS Google Scholar * Jonas JB, Kreissig I, Degenring R . Intraocular pressure after intravitreal injection of
triamcinolone acetonide. _Br J Ophthalmol_ 2003; 87: 24–27. Article CAS Google Scholar * Bakri SJ, Beer PM . The effect of intravitreal triamcinolone acetonide on intraocular pressure.
_Ophthalmic Surg Lasers Imaging_ 2003; 34: 386–390. PubMed Google Scholar * Benz MS, Murray TG, Dubovy SR, Katz RS, Eifrig CW . Endophthalmitis caused by _Mycobacterium chelonae_ abscessus
after intravitreal injection of triamcinolone. _Arch Ophthalmol_ 2003; 121: 271–273. Article Google Scholar * Jonas JB, Kreissig I, Degenring RF . Endophthalmitis after intravitreal
injection of triamcinolone acetonide. _Arch Ophthalmol_ 2003; 121: 1663–1664. Article Google Scholar * Jonas JB, Bleyl U . Morphallaxia-like ocular histology after intravitreal
triamcinolone acetonide. _Br J Ophthalmol_ 2004, (in print). * Ishibashi T, Miki K, Sorgente N, Patterson R, Ryan SJ . Effects of intravitreal administration of steroids on experimental
subretinal neovascularization in the subhuman primate. _Arch Ophthalmol_ 1985; 103: 708–711. Article CAS Google Scholar * Wilson CA, Berkowitz BA, Sato Y, Ando N, Handa JT, de Juan Jr E .
Treatment with intravitreal steroid reduces blood–retinal barrier breakdown due to retinal photocoagulation. _Arch Ophthalmol_ 1992; 110: 1155–1159. Article CAS Google Scholar * Antoszyk
AN, Gottlieb JL, Machemer R, Hatchell DL . The effects of intravitreal triamcinolone acetonide on experimental pre-retinal neovascularization. _Graefes Arch Clin Exp Ophthalmol_ 1993; 231:
34–40. Article CAS Google Scholar * Penfold PL, Wen L, Madigan MC, King NJ, Provis JM . Modulation of permeability and adhesion molecule expression by human choroidal endothelial cells.
_Invest Ophthalmol Vis Sci_ 2002; 43: 3125–3130. Google Scholar * Penfold PL, Wong JG, Gyory J, Billson FA . Effects of triamcinolone acetonide on microglial morphology and quantitative
expression of MHC-II in exudative age-related macular degeneration. _Clin Exp Ophthalmol_ 2001; 29: 188–192. Article CAS Google Scholar * Penfold PL, Wen L, Madigan MC, Gillies MC, King
NJ, Provis JM . Modulation of permeability and adhesion molecule expression by human choroidal endothelial cells. _Invest Ophthalmol Vis Sci_ 2002; 43: 3125–3130. Google Scholar * McCuen II
BW, Bessler M, Tano Y, Chandler D, Machemer R . The lack of toxicity of intravitreally administered triamcinolone acetonide. _Am J Ophthalmol_ 1981; 91: 785–788. Article Google Scholar *
Hida T, Chandler D, Arena JE, Machemer R . Experimental and clinical observations of the intraocular toxicity of commercial corticosteroid preparations. _Am J Ophthalmol_ 1986; 101: 190–195.
Article CAS Google Scholar * Kwak HW, D’Amico DJ . Evaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. _Arch Ophthalmol_ 1992; 110:
259–266. Article CAS Google Scholar * Kivilcim M, Peyman GA, El-Dessouky ES, Kazi AA, Cheema R, Hegazy H . Retinal toxicity of triamcinolone acetonide in silicone-filled eyes. _Ophthalmic
Surg Lasers_ 2000; 31: 474–478. CAS Google Scholar * Degenring RF, Kamppeter B, Kreissig I, Jonas JB . Morphologic and functional changes after intravitreal triamcinolone acetonide for
retinal vein occlusion. _Acta Ophthalmol_ 2003; 81: 548–550. Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Ophthalmology and Eye Hospital,
Faculty for Clinical Medicine, Mannheim Ruprecht-Karls-University, Heidelberg, Germany J B Jonas, I Akkoyun, B Kamppeter, I Kreissig & R F Degenring Authors * J B Jonas View author
publications You can also search for this author inPubMed Google Scholar * I Akkoyun View author publications You can also search for this author inPubMed Google Scholar * B Kamppeter View
author publications You can also search for this author inPubMed Google Scholar * I Kreissig View author publications You can also search for this author inPubMed Google Scholar * R F
Degenring View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to J B Jonas. ADDITIONAL INFORMATION Proprietary interest:
none. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jonas, J., Akkoyun, I., Kamppeter, B. _et al._ Branch retinal vein occlusion treated by
intravitreal triamcinolone acetonide. _Eye_ 19, 65–71 (2005). https://doi.org/10.1038/sj.eye.6701395 Download citation * Received: 01 October 2003 * Accepted: 10 November 2003 * Published:
23 April 2004 * Issue Date: 01 January 2005 * DOI: https://doi.org/10.1038/sj.eye.6701395 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * branch
retinal vein occlusion * triamcinolone acetonide * intraocular steroids * intravitreal steroids * intraocular pressure * cystoid macular oedema
