Non-mendelian segregation and variable penetrance of colour genes in the polymorphic butterfly danaus chrysippus (l. )
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Non-Mendelian segregation involving three colour gene loci in the butterfly _Danaus chrysippus_ is reported. In each of the four affected broods, two of the four phenotypes expected
in the progeny were absent in both sexes. Operation of a system of balanced recessive lethals is ruled out by the low egg–adult mortality recorded. All gametes were produced in expected
ratios and were viable. The results are interpreted to be consistent with an inherited prezygotic incompatibility mechanism which operates at the time of fertilization. Variable penetrance
of the recessive _a_ and _c_ alleles in heterozygotes is significantly influenced, in the former case by sex, and in the latter by epistatic interactions with colour genes at the _A_ and _B_
loci. The combination of non-Mendelian segregation, variable incomplete dominance and epistatic interactions with other colour genes and sex supports the hypothesis, hitherto based largely
on biogeographical evidence, that the polymorphic _D. chrysippus_ population at Dar es Salaam, Tanzania may have a hybrid origin. SIMILAR CONTENT BEING VIEWED BY OTHERS A LARGE AND DIVERSE
AUTOSOMAL HAPLOTYPE IS ASSOCIATED WITH SEX-LINKED COLOUR POLYMORPHISM IN THE GUPPY Article Open access 09 March 2022 SIMPLE INHERITANCE OF COLOR AND PATTERN POLYMORPHISM IN THE STEPPE
GRASSHOPPER _CHORTHIPPUS DORSATUS_ Article Open access 16 April 2021 GENE-POOR Y-CHROMOSOMES SUBSTANTIALLY IMPACT MALE TRAIT HERITABILITIES AND MAY HELP SHAPE SEXUALLY DIMORPHIC EVOLUTION
Article 10 February 2023 INTRODUCTION _Danaus chrysippus_ is a widespread and abundant butterfly which is distributed throughout the Old World tropics in open habitats. In Uganda, Kenya,
Tanzania and neighbouring countries, but not elsewhere, an extensive range of polymorphic forms is sympatric and they interbreed freely (Owen & Chanter, 1968; Smith, 1975a, 1980; Gordon,
1984). Unusual sex ratios have been described (Owen & Chanter, 1968; Smith, 1975b; Gordon, 1984), both in the field and within broods; less commonly, non-Mendelian segregations for
colour genes have been reported, some of which are associated with all-female broods (Smith, 1976; Gordon, 1984) and others not (Owen & Chanter, 1968; Gordon, 1984). Moreover, there are
several reports of morph ratios in the field which differ between the sexes (Smith, 1980; Smith et al., 1993, 1997). Here, I describe a type of non-Mendelian segregation not hitherto
reported in _D. chrysippus_ or, to my knowledge, in any other butterfly, together with evidence for variable penetrance of two recessive alleles contingent upon, in one case sex, and in the
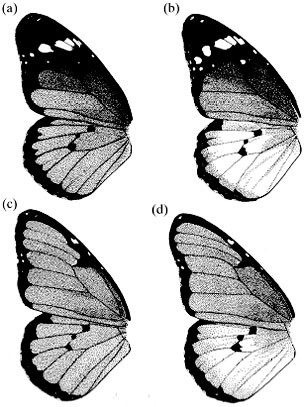
other, epistatic interaction with two other colour gene loci. The basic colour genetics of _D. chrysippus_ is now fairly well known (Clarke et al., 1973; Smith, 1975a, 1980) (Fig. 1).
METHODS The founder pair for this study was taken _in copula_ in the field at Dar es Salaam, Tanzania. Eggs were obtained in a sleeve on a branch of the foodplant _Calotropis gigantea_. All
the females used are direct descendants of the founder female but wild males were sometimes introduced to obtain required matings. Pairing took place in an outdoor insectary from which mated
females were removed and placed in sleeves. All broods were reared in separate cages and fed on _C. gigantea._ RESULTS NON-MENDELIAN SEGREGATION (BROODS 82–85) The broods used in this study
are shown in Table 1. The founder brood 82 (Table 2) shows some unusual features which stimulated the remainder of the breeding programme. Segregation is 16:11 (1:1) for orange (_bb_):brown
(_Bb_) (omitted from Table 2 for reasons of clarity), 20:7 (3:1) for _dorippus_ (_A–C–_): _albinus_ (_aaC–_) and 10:17 for sex. The most obvious anomaly is the absence of _cc_ forewing
phenotypes (_chrysippus_ or _alcippus_) from the progeny; because both parents are visibly _Cc_ (_transiens_), a 3:1 segregation, including 1/4×27=6.75 _cc_, is expected. Moreover, the eight
_Cc_ heterozygotes with penetrant _c_ (_transiens_) are distributed nonrandomly with respect to the _A_ locus, as seven of them have the visually determinable genotypes _aa_ _Bc_/_bC_ or
_aa_ _bC_/ _bc_, whereas the _aa_ _bC_/ _bC_ and _aa_ _Bc_ _bc_ genotypes are both missing. Assuming random assortment for the _A_ and _C_ loci, H0 for a 9:3:4 segregation (with the _A–cc_
and _aacc_ classes amalgamated) is rejected (χ22=9.02; 0.02> _P_≲0.01). Analysed in four classes without pooling, the combined segregation for the _A_ and _C_ loci has an exact
probability (two-tailed) of 1.8×10−5. Association of the _A_ and _bC_ chromosomes (_n_=12) and the _a_ and _bc_ chromosomes (_n_=4), combined with the absence of the alternative
arrangements, _a_ with _bC_ and _A_ with _bc_, has a highly significant exact probability (two-tailed) of 1.1×10−5. By deduction, the array of gamete pairings found in brood 82 (Table 3)
suggests that half of those predicted, assuming random assortment, are absent (four cells are in doubt because penetrance of the _a_ and _c_ alleles is impossible to estimate), whereas all
observed pairings exceed expectation. And yet, it is clear that all gametes, with the possible exception of _A/bc_ in males, are produced and functional. Three F2 broods (Table 4) were bred
from the brood 82 progeny to generate better data for the interpretation of the non-Mendelian segregations observed and, as all selected parents were _aa_, to reduce genetic noise. Parents
were selected for their visually identifiable _B-_ and _C-_locus genotypes. Furthermore, brood size was maximized and careful egg–adult mortality records kept. Not only were all the female
parents sibs, but the same male sired two broods. The segregation expected with independent assortment is 1:1:1:1 for _Bc_/ _bC_, _bC_/ _bC_, _bC_/ _bc_ and _Bc_/ _bc_: however, the large
progenies probably comprise only two of the expected four genotypes. Yet it is clear in all these broods that both parents contribute both their _BC_ chromosomes equally to offspring of both
sexes. How can I be sure that all the orange _albinus_ should be scored as _aa_ _bC_/ _bc_ rather than _aa_ _bC_/ _bC_? Two facts support the interpretation that the latter genotype is
missing. First, the penetrance of _c_ in these broods is slightly above average and homogeneous, 47% in _bC_/ _bc_ and 81.9% in _Bc_/ _bC_, compared to 45% (_n_=129) and 74.4% (_n_=173),
respectively, in known backcross progenies from Tanzania (Smith, 1980); secondly, all three brood segregations fit a 1:1 expectation; there is no surplus of orange (_bb_) individuals which
would be expected if both _bC_/ _bc_ and _bC_/ _bC_ genotypes were recovered. A parsimonious interpretation is that the _bC_/ _bC_ genotype is missing, as is the easily recognized _Bc_/ _bc_
genotype. Note that both these genotypes are also absent from the parental brood 82. PENETRANCE OF THE C ALLELE IN CC HETEROZYGOTES Penetrance of the _a_ and _c_ alleles clearly varies with
genetic background in broods 82–85. Broods 87–95 were reared to provide larger samples for estimating penetrance against a wider variety of genetic backgrounds. The results for the _C_
locus (Table 5) show that penetrance of _c_ in _Cc_ genotypes is higher in a _Bb_ (72.3%) than a _bb_ (44.9%) background and in _aa_ (67.6%) compared to _A_- (49.0%) genotypes. Because the
χ2-value for interaction is not significant, the two effects are probably additive, the _aaBbCc_ genotype showing penetrance of 83.3%, compared to 0% for _A–bbCc_ (this sample is small
(_n_=12) and the estimate atypically low). The highly significant interaction between the _A-_ and _B_-locus segregations is caused by the non-Mendelian effects described above for broods
82–85. Penetrance is probably not generally affected by sex (χ21=3.44, 0.1> _P_≲0.05) although the influence of sex is significantly heterogeneous (χ23=10.99, 0.02> _P_≲0.01) and
requires further investigation. PENETRANCE OF THE A ALLELE IN AA HETEROZYGOTES In contrast to the _C_ locus, penetrance of _a_ in _Aa_ heterozygotes (Table 6) is strongly influenced by sex,
as it is significantly higher (62.5%) in males than females (25.5%). It is not, however, affected by _C_-locus genotype; a possible _B_-locus effect cannot be tested with these data as
broods 87–88 are entirely brown. DISCUSSION NON-MENDELIAN SEGREGATION The segregations in broods 82–85, and especially the last three, suggest at first glance the operation of a balanced
recessive lethal system. This interpretation is, however, effectively ruled out by the mortality data for broods 83–85 Table 4 which show that the mean egg–adult mortality of 26.4% is well
below the minimum of 50% expected if two classes from a 1:1:1:1 segregation were to suffer total mortality. Brood mortality is, moreover, well below the average of 39.8% (_n_=52 broods
measured) for bisexual broods reared by me in Tanzania and is homogeneous across the three broods. Preoviposition mortality or reabsorption of eggs probably never occurs in butterflies
because eggs are fertilized singly, immediately before laying, as they pass the opening from the bursa copulatrix. Usually females carry only one fertilized egg at a time. In broods 83–85
(Table 4), all gametes are viable and apparently produced by both sexes in equal numbers. As both types of sperm are equally recovered in the progeny, sperm competition is ruled out.
Compatible pairings are _Bc_ with _bC_ and _bC_ with _bc_ and incompatible pairings _bC_ with _bC_ and _Bc_ with _bc_. As these four pairings behave, in terms of compatibility, identically
among the three F2 broods and the parental brood 82, the mechanism must be inherited. Moreover, because broods 83 and 85, on the one hand, and 84 on the other, are reciprocal crosses,
compatibility must be irrespective of whether the haploid genomes involved are carried by sperm or eggs. These results suggest the operation of a prezygotic isolating mechanism which
operates at the point of fertilization. It may be relevant that the parents of brood 82 are heterozygous for three genes with alleles which are at fixation in the allopatric subspecies
_chrysippus_ (_AAbbcc_) (North Africa and Asia), _liboria (AABBcc_) (southern Africa), _alcippus_ (_aabbcc_) (West Africa) and _dorippus_ (_AAbbCC_) (north-east Africa), all of which overlap
and interbreed in Tanzania. Recombination through independent _A_/ _BC_ assortment in both sexes and _B_/ _C_ cross-over in males has resulted in many phenotypes (_transiens_ (–––– _Cc_),
brown _dorippus_ (_A–B–C–_) and _albinus_ (_aa––C–_) which are confined to the postulated hybrid zone. It may be that prezygotic isolation, together with disturbed sex ratios, indicates
incipient speciation involving two or more of these subspecies. VARIABLE PENETRANCE OF THE A AND C ALLELES Penetrance of the _a_ allele is apparently unaffected by genotype at the _C_ locus
and a possible effect from the _B_ locus remains to be investigated. The partial sex-limitation of penetrance is possibly a stage in the evolution of full sex-limited inheritance which is
rather common in butterflies (though not in Batesian models such as _D. chrysippus_), e.g. _Papilio dardanus_ (Clarke & Sheppard, 1963) and _P. memnon_ (Clarke et al., 1968), _Hypolimnas
misippus_ (Smith & Gordon, 1987; Gordon & Smith, 1989) and _H. bolina_ (Clarke & Sheppard, 1975), _Colias_ and _Catopsilia_ species, although in all these cases it is the male
in which gene penetrance is suppressed. However, in _Pseudacraea eurytus_ (Carpenter, 1949; Owen & Chanter, 1972) sex-limited phenotypes occur in both sexes. Expressivity of _a_ in the
_Aa_ genotype is very variable; at the extremes, individuals with, on the one hand, a few white scales lining the hindwing veins and, on the other, a white patch scarcely smaller than found
in _aa_ individuals, suggests that evolution of dominance has not occurred. It is my impression that, not only penetrance but also expressivity (which has not been measured), is higher in
males. Owen & Chanter (1968), working in Uganda, found that the frequency of weak _alcippus_ (_Aa_) was significantly lower in the field than in a sample reared from wild-collected
larvae. They suggested that selection for distinctiveness between the polymorphic forms, which improves mimetic resemblance to _H. misippus_ and _Acraea encedon_ (now known to include _A.
encedana_ (Owen et al., 1994)), might be responsible. If this is the case, selection for improved mimicry is stronger in the female than the male. Interaction of the _C_ and _c_ alleles in
_C_-locus heterozygotes differs from the _A_-locus interaction in that the phenotype of the _transiens_ (_Cc_) form (with penetrant _c_) is much closer to _dorippus_ than to _chrysippus_,
indeed so much so that it is unlikely that any predator would discriminate between _transiens_ and _dorippus_. In this case dominance may have evolved, an hypothesis which is testable by
crossing distantly allopatric races. On the other hand, the near dominance of the _C_ allele may be a simple consequence of the biochemistry of gene action. The penetrance data at both _A_
and _C_ loci, showing as they do multiple epistatic interactions with sex and other loci, which in turn have variable penetrance, serve to emphasize the practical impossibility on present
knowledge of measuring allele frequencies at any of the _A_, _B_ or _C_ loci directly from field samples. Moreover, the occurrence of non-Mendelian segregations for phenotype, not only of
the type described here, but also associated with all-female broods (Smith, 1975b, 1976; Gordon, 1984) and bisexual broods (Gordon, 1984) strongly suggests that intergeneration changes of
colour gene frequencies are at present hard to predict. The highly complex set of interactions in the polymorphic population at Dar es Salaam has probably arisen as a result of the recent
admixture of several well-differentiated subspecies to form an extensive hybrid zone. REFERENCES * Carpenter, G.. D. H. (1949). _Pseudacraea eurytus_ (L.) (Lep. Nymphalidae): a study of a
polymorphic mimic in various degrees of speciation. _Trans R Ent Soc Lond_, 100: 71–133. Article Google Scholar * Clarke, C. A. and Sheppard, P. M. (1963). Interactions between major genes
and polygenes in the determination of mimetic patterns of _Papilio dardanus_. _Evolution_, 17: 404–413. Article Google Scholar * Clarke, C. A. and Sheppard, P. M. (1975). The genetics of
the mimetic butterfly _Hypolimnas bolina_ (L.). _Phil Trans R Soc_, 272: 229–265. Article CAS Google Scholar * Clarke, C. A., Sheppard, P. M. and Thornton, I. W. B. (1968). The genetics
of the mimetic butterfly _Papilio memnon_ L. _Phil Trans R Soc_, 254: 37–89. Article Google Scholar * Clarke, C. A., Sheppard, P. M. and Smith, A. G. (1973). The genetics of fore and
hindwing colour in crosses between _Danaus chrysippus_ from Australia and from Sierra Leone (Danaidae). _J Lepidopt Soc_, 27: 73–77. Google Scholar * Gordon, I. J. (1984). Polymorphism of
the tropical butterfly _Danaus chrysippus_ L. in Africa. _Heredity_, 53: 583–593. Article Google Scholar * Gordon, I. J. and Smith, D. A. S. (1989). Genetics of the mimetic African
butterfly _Hypolimnas misippus_: hind-wing polymorphism. _Heredity_, 63: 409–425. Article Google Scholar * Lewis, B. N. (1962). On the analysis of interaction in multi-dimensional
contingency tables. _J R Statist Soc_, 125: 88–117. Google Scholar * Owen, D. F. and Chanter, D. O. (1968). Population biology of tropical African butterflies. 2. Sex ratio and polymorphism
in _Danaus chrysippus_ L. _Rev Zool Bot Afr_, 78: 81–97. Google Scholar * Owen, D. F. and Chanter, D. O. (1972). Polymorphic mimicry in a population of the African butterfly, _Pseudacraea
eurytus_ (L.) (Lep. Nymphalidae). _Ent scand_, 3: 258–266. Article Google Scholar * Owen, D. F., Smith, D. A. S., Gordon, I. J. and Owiny, A. M. (1994). Polymorphic Müllerian mimicry in a
group of African butterflies: a re-assessment of the relationship between _Danaus chrysippus_, _Acraea encedon_ and _Acraea encedana_ (Lepidoptera: Nymphalidae). _J Zool Lond_, 232: 93–108.
Article Google Scholar * Smith, D. A. S. (1975a). Genetics of some polymorphic forms of the African butterfly _Danaus chrysippus_ L. (Lepidoptera: Danaidae). _Ent scand_, 6: 134–144.
Article Google Scholar * Smith, D. A. S. (1975b). All-female broods in _Danaus chrysippus_ L. and their ecological significance. _Heredity_, 34: 363–371. Article CAS Google Scholar *
Smith, D. A. S. (1976). Evidence for autosomal meiotic drive in the butterfly _Danaus chrysippus_. _Heredity_, 36: 139–142. Article CAS Google Scholar * Smith, D. A. S. (1980). Heterosis,
epistasis and linkage disequilibrium in a wild population of the polymorphic butterfly _Danaus chrysippus_. _Zool J Linn Soc_, 69: 87–109. Article Google Scholar * Smith, D. A. S. and
Gordon, I. J. (1987). The genetics of the polymorphic tropical butterfly _Hypolimnas misippus_: the classification of phenotypes and the inheritance of forms _misippus_ and _inaria_.
_Heredity_, 59: 467–475. Article Google Scholar * Smith, D. A. S., Owen, D. F., Gordon, I. J. and Owiny, A. M. (1993). Polymorphism and evolution in the butterfly _Danaus chrysippus_ (L.)
(Lepidoptera: Danainae). _Heredity_, 71: 242–251. Article Google Scholar * Smith, D. A. S., Owen, D. F., Gordon, I. J. and Lowis, N. K. (1997). The butterfly _Danaus chrysippus_ (L.) in
East Africa: polymorphism and morph-ratio clines within a complex, extensive and dynamic hybrid zone. _Zool J Linn Soc_, 120: 51–78. Article Google Scholar Download references
ACKNOWLEDGEMENTS I am grateful to the University of Dar es Salaam for providing the facilities for carrying out this work. Dr Derek Whiteley drew Fig. 1 AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Natural History Museum, Eton College, Windsor, SL4 6EW, Berkshire, UK David A S Smith Authors * David A S Smith View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to David A S Smith. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Smith, D. Non-Mendelian
segregation and variable penetrance of colour genes in the polymorphic butterfly _Danaus chrysippus_ (L.). _Heredity_ 80, 474–480 (1998). https://doi.org/10.1046/j.1365-2540.1998.00314.x
Download citation * Received: 16 April 1997 * Published: 01 April 1998 * Issue Date: 01 April 1998 * DOI: https://doi.org/10.1046/j.1365-2540.1998.00314.x SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * colour genes * _Danaus chrysippus_ * epistasis * non-Mendelian segregation * penetrance * prezygotic isolation
