Pre-irradiation of mouse mammary gland stimulates cancer cell migration and development of lung metastases
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT BACKGROUND: In most patients with breast cancer, radiotherapy induces inflammation that is characterised by an increase of promigratory factors in healthy tissues surrounding the
tumour. However, their role in the emergence of the migration phenotype and formation of metastases is still unclear. METHODS: A single mammary gland of BALB/c mice was irradiated with four
doses of 6 Gy given at a 24-h interval. After the last session of irradiation, treated and control mammary glands were either collected for quantification of promigratory and proinflammatory
factors or were implanted with fluorescent ubiquitination-based cell cycle indicator (FUCCI)-expressing mouse mammary cancer D2A1 cells. The migration of cancer cells in the mammary glands
was monitored by optical imaging. On day 21, mammary tumours and lungs were collected for histology analyses and the quantification of metastases. RESULTS: Pre-irradiation of the mammary
gland increased by 1.8-fold the migration of cancer cells, by 2-fold the quantity of circulating cancer cells and by 2.4-fold the number of lung metastases. These adverse effects were
associated with the induction of interleukin-6 (IL-6) and cyclooxygenase-2 (COX-2). CONCLUSION: The emergence of the metastasis phenotype is believed to be associated with the accumulation
of mutations in cancer cells. Our results suggest an alternative mechanism based on promigratory factors from irradiated mammary glands. In clinic, the efficiency of radiotherapy could be
improved by anti-inflammatory agents that would prevent the stimulation of cancer cell migration induced by radiation. SIMILAR CONTENT BEING VIEWED BY OTHERS RADIATION-INDUCED AMPHIREGULIN
DRIVES TUMOUR METASTASIS Article 14 May 2025 RADIATION EXPOSURE ELICITS A NEUTROPHIL-DRIVEN RESPONSE IN HEALTHY LUNG TISSUE THAT ENHANCES METASTATIC COLONIZATION Article 24 February 2022
INTERLEUKIN-6 TRANS-SIGNALING IS A CANDIDATE MECHANISM TO DRIVE PROGRESSION OF HUMAN DCCS DURING CLINICAL LATENCY Article Open access 05 October 2020 MAIN Radiotherapy is an important part
of breast cancer treatment. This modality can completely cure the disease or eliminate a large number of cancer cells, and it can reduce the recurrence rate and increase the overall survival
of patients. It is worth noting that the total radiation dose is limited by the tolerance of surrounding normal tissues and is not meant to eradicate all cancer cells scattered in the
breast, but rather to optimise long-term results with minimal adverse effects. Consequently, women still have a nonnegligible risk of breast cancer death after radiotherapy (Clarke et al,
2005). Clinicians strive to increase the effectiveness of radiotherapy within acceptable limits of host toxicity, which aim to minimise adverse effects such as inflammation of normal tissues
potentially causing fibrosis or dermatitis. Radiotherapy is recognised to trigger an inflammatory response (Gallet et al, 2011). This inflammation is characterised by an increase of
cytokines, angiogenic factors, adhesion molecules and matrix metalloproteinases (MMPs) (Rodemann and Blaese, 2007). It is also known that chronic inflammation increases the risk of
developing several types of cancer, including breast cancer (Mantovani et al, 2008). Observations also suggest that radiation might promote the invasiveness of breast cancer cells (Madani et
al, 2008). For instance, we recently reported that mouse thighs that were pre-irradiated increased the invasiveness of implanted mammary cancer cells (Lemay et al, 2011). Another study
demonstrated that radiation promoted changes in the mammary gland stromal microenvironment that contributed to the tumourigenic potential of breast cancer cells (Barcellos-Hoff and Ravani,
2000). However, the functional roles of the promigratory molecules induced by radiation during the early phase of the metastatic cascade remain unresolved. A better understanding of the
alleged prometastatic properties of radiation could contribute to the development of new therapeutic modalities to prevent these undesirable effects. The evidence linking the
microenvironment to tumour progression is growing (Goldberg and Schwertfeger, 2010). In an effort to determine the role of irradiation in the progression of breast cancer, we pre-irradiated
a mouse mammary gland and then implanted triple-negative mammary carcinoma cells D2A1. This protocol allowed us to specifically assess whether inflammation induced by radiation could
stimulate the progression of cancer cells. Our procedure had the advantage of defining the mechanisms involved and eliminating confounding effects that could occur by irradiating the tumour
and the mammary gland at the same time. Some examples of confounding effects are the selection of cancer cells more likely to migrate or the induction of mutations that would increase the
aggressiveness of tumour cells. Although the mammary gland radiation-induced stromal effect and carcinogenesis have been studied (Barcellos-Hoff, 2010), we are, to our knowledge, the first
to investigate a preclinical model of breast cancer recurrence following standard fractionated radiotherapy. Our innovative mouse model of triple-negative breast cancer cell migration is a
step forward in the understanding of metastatic breast cancer. In this study, mouse mammary glands were pre-irradiated that stimulated the migration of mammary cancer cells at the primary
site of implantation, increased the number of circulating cancer cells and promoted lung metastases. These adverse effects of radiation were associated with the increased expression in the
irradiated tissue of the key pro-inflammatory factors, cyclooxygenase-2 (COX-2) and interleukin-6 (IL-6). MATERIALS AND METHODS CELL CULTURE The mouse D2A1 cancer cells, kindly provided by
Dr Ann F Chambers (University of Western Ontario, London, ON, Canada), are derived from a spontaneous mammary tumour in a BALB/c mouse (Rak et al, 1992). These cells were maintained in a 5%
CO2 humidified incubator at 37 °C in modified Eagle’s medium (MEM) (Sigma-Aldrich, Oakville, ON, Canada) supplemented with 10% fetal bovine serum (Wisent, St Bruno, QC, Canada), 2 mM
glutamine, 1 mM sodium pyruvate, 100 units per ml penicillin and 100 _μ_ M streptomycin. MIGRATION CAPACITY OF D2A1 CELLS ASSESSED IN INVASION CHAMBERS For the invasion assay, BALB/c 3T3
fibroblasts (2.5 × 104) were seeded with MEM supplemented with 10% FBS in 24-well plates. After 20 h, the cell culture medium was replaced with MEM supplemented with 0.1% bovine serum
albumin (BSA) following two rinses in PBS. Cells were then irradiated using a 60Co source (Gammacell 220, Nordion, Canada) at a dose of 5 Gy. Sham-irradiated cells were used as a control.
The fibroblast conditioned media were used as a chemoattractant in the lower compartment of the invasion chambers (Becton Dickinson Biosciences, Bedford, MA, USA). Invasion chambers coated
with Matrigel (artificial basement membrane) were rehydrated with 1 ml MEM 0.1% BSA for 2 h at 37 °C. Nonirradiated D2A1 mouse mammary cancer cells harvested with Cell Dissociation Solution
(Sigma-Aldrich) were added (4 × 104) to the upper compartment of the invasion chambers 24 h after irradiation of the BALB/c 3T3 cells. Mouse mammary cancer cells that had passed across the
Matrigel and the porous membrane 24 h later were fixed, stained with crystal violet and counted under the microscope. Results were reported as radiation-enhancement ratio. Each experiment
was performed in triplicate and repeated three times. GENERATION OF D2A1 CELLS EXPRESSING THE FLUORESCENT UBIQUITINATION-BASED CELL CYCLE INDICATOR (FUCCI) PROTEINS Genes encoding for the
FUCCI proteins were introduced into the D2A1 cells to allow the detection and assessment of their cell cycle state by optical imaging. Replication-defective, self-inactivating CSII-EF-MCS
lentiviral vectors encoding for Cdt1 and the Geminin E3 ligases substrates fused, respectively, to the red monomeric version of the Kusabira Orange (mKO2-hCdt1) and the green monomeric Azami
Green (mAG-hGem) fluorescent proteins, which were generously provided by Dr Asako Sakaue-Sawano (Brain Science Institute, RIKEN, Wako, Saitama, Japan). Red and green fluorescence are
respectively markers of cells within the G1 (red) and S/G2/M (green) phases of the cell cycle (Sakaue-Sawano et al, 2008). Each construct was co-transfected with plasmids encoding for the
lentiviral packaging proteins (plp1, plp2 and plp/VSVG) in human embryonic kidney 293T cells using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen, Burlington,
ON, Canada). After a 48-h incubation, lentivirus-containing supernatants were harvested and filtered with a 0.45-_μ_m filter, and then kept at −80 °C until further use. D2A1 cell population
expressing the FUCCI proteins were generated following a triple sequential infection for each fluorescent protein (Wu et al, 2010). MAMMARY GLAND PRE-IRRADIATION AND INJECTION OF D2A1
FUCCI-EXPRESSING CELLS The experimental protocols were approved by the institutional ethics committee and conformed to the regulations of the Canadian Council on Animal Care. Female retired
breeder BALB/c mice (18–24 weeks old) were obtained from Charles River (Raleigh, NC, USA). Animals were anaesthetised with 3% isoflurane and then immobilised with a stereotactic mouse frame
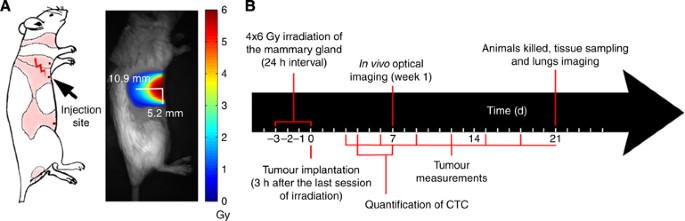
adapted to dock on the Leskell Gamma Knife Perfexion (Elekta, Stockholm, Sweden). The third right mammary gland was irradiated by an energy deposition of elliptical shape (Figure 1A).
Anaesthetised mice were irradiated at a dose rate of 1.33 Gy min−1 to a total of 6 Gy during each of the 4 fractions at 24 h intervals. Based on dosimetry performed by our institutional
medical physicist team, this protocol provided a biological effective dose (BED) comparable to the standard clinical regimen of 20 × 2.25 Gy, without having to perform daily anaesthesia over
20 days that would be lethal in mice. Regarding the nonirradiated mammary glands, they received a residual dose of <1%. To determine whether pre-irradiation of the mammary gland
stimulated the migration of mouse mammary cancer cells, D2A1 FUCCI-expressing cells (106 per 100 _μ_l PBS) were injected 3 h after the last irradiation into the pre-irradiated (right side)
and unirradiated (control, left side) mammary glands. Mouse mammary carcinoma cells were also implanted into the mammary glands of sham-irradiated mice. The tumour volume was measured every
3 days by external caliper measurements and calculated with the formula: _V_ (mm3)=_π_/6 × _a_ (mm) × _b_2 (mm2), where ‘_a_’ and ‘_b_’ are the largest and smallest perpendicular tumour
diameters, respectively (Balin-Gauthier et al, 2006). In other experiments, the D2A1 FUCCI-expressing cells (106 per 100 _μ_l PBS) were instead injected intravenously via the tail vein of
sham (_n_=4) and pre-irradiated mice (_n_=4). After 9 days, these animals were killed and their lungs were processed to quantify the number of metastases. The number and the diameter of lung
metastases were quantified using the CellProfiler 2.0.0 software. Parameters were set to an intensity-based identification method on images containing a dense amount of cancerous growth or
a shape-based identification method on images containing a sparse amount of metastasis, using the fixed parameters. Figure 1B summarises the chronological order of irradiation and the
handling of animals. _IN VIVO_ AND _IN SITU_ OPTICAL IMAGING The migration of D2A1 FUCCI-expressing cancer cells in the mammary gland was monitored with an animal optical imager (QOS Imager,
Quidd S.A.S., Val de Reuil, France). Mice were anaesthetised with ketamine/xylazine (87 : 13 mg ml−1 at 1 mg kg−1). A bright field image of the mice was taken and then the appropriate
filters were selected for red and green fluorescent image acquisition (mKO2, _λ_ex=472/30, _λ_em=536/40; mAG, _λ_ex=531/40, _λ_em=593/40). The three images acquired were merged for future
analysis. Distances of D2A1 cell migration in irradiated and nonirradiated mammary glands were measured to determine the radiation-enhancement ratio. Migration was quantified with ImageJ
(NIH, USA) as the distance from the nipple (physical landmark for injection site) to the end of fluorescent smear. On day 21, mice were killed, and tumour and lung specimens were removed
(sham; _n_=12 sham, irradiated; _n_=9). Fluorescence images of the lungs were acquired and lungs metastases were quantified as described above. HISTOLOGY Mammary tumours and lung specimens
containing D2A1 FUCCI-expressing cancer cells were collected and immediately frozen in a solution of Optimum Cutting Temperature (OCT; Electron Microscopy Sciences, Hatfield, PA, USA).
Cryosections of 3 _μ_m were cut using a Leica CM3050 Microsystems cryostat (GmbH, Wetzlar, Germany). Slides were dried for 30 min at 37 °C and then stored at −80 °C until further use. The
fluorescence emitted by the D2A1 cells was recorded using the FSX100 Bio Imaging Navigator microscope (Olympus, Center Valley, PA, USA) equipped with band pass filters (Chroma Technology
Corp., Bellows Falls, VT, USA) for fluorescein isothiocyanate (FITC; _λ_ex=480/30, _λ_em=535/40) or tetramethylrhodamine isothiocyanate (TRITC; _λ_ex=560/40, _λ_em=630/60). To calculate the
ratio of red-to-green fluorescence intensity of cells in the tumours, the entire slide was scanned (magnification × 42) and every image was quantified for red and green signals.
QUANTIFICATION OF INFLAMMATORY AND PRO-MIGRATORY FACTORS In different groups of irradiated mice (_n_=6 per group), animals were killed at 4, 7 or 24 h post irradiation, and their mammary
glands were removed and snap frozen. Prostaglandin D2 (PGD2) and E2 (PGE2) levels were quantified by liquid chromatography/tandem mass spectrometry (LC-MS/MS) (Yang et al, 2002). The mRNA
levels of COX-2, 15-hydroxyprostaglandin dehydrogenase (15-PGDH), IL-1_β_, IL-6, membrane type 1 metalloprotease (MT1-MMP), phospholipase A2 (PLA2), transforming growth factor-_β_1
(TGF-_β_1) and tumour necrosis factor-_α_ (TNF-_α_) were determined by quantitative real-time PCR(qPCR) in irradiated and contralateral nonirradiated mammary glands 6 h after the last
session of irradiation (_n_=6). Tissues were submerged in RNAlaterTM (Qiagen Inc., Toronto, ON, Canada) stored at 4 °C for 24 h and then at −80 °C. Total RNA extractions, reverse
transcription, primer dilutions and PCR reactions were made with the FastStart Universal SYBR Green Master mix (Roche Diagnostics, QC, Canada). The following cycling conditions were used: 10
min at 95 °C, and then 50 cycles of 15 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C. Relative expression levels were calculated using the qBASE framework and normalised to the mouse UBC,
HPRT1 and GAPDH housekeeping genes (Desmarais et al, 2012). The sequences of the primers that were used are listed in Supplementary Table 1 in the Supplementary Materials. MMP-2 and MMP-9
levels were analysed by zymography in mammary glands and tumour tissues in either irradiated or sham animals, using methods previously described (_n_=6) (Lemay et al, 2011) and were also
confirmed by immunohistochemistry (IHC) on 3 _μ_m paraffin-embedded tissues. The signal revelation of MMP-2 (Thermo Scientific, IL, USA) and MMP-9 (Antibodies-Online Inc., GA, USA)
antibodies was realised using an anti-rabbit HRPO secondary antibody (dilution 1 : 1000; AbD Serotec, UK) and the Dako EnVision HRP system (Carpinteria, CA, USA). Tissues were counterstained
with methyl green. CIRCULATING TUMOUR CELLS (CTC) Blood samples were collected from the lateral saphenous vein of the sham (_n_=3) and pre-irradiated (_n_=3) mice at 4 and 7 days after the
injection of D2A1 FUCCI-expressing cells in the mammary glands. Samples diluted 1 : 10 in PBS were spread in a Petri dish. The presence of CTC in each blood sample was quantified by
fluorescence microscopy from 10 images of representative areas (magnification × 100) that were acquired as described above. STATISTICAL ANALYSIS Experimental data are presented as
mean±s.e.m. Statistical analyses were performed using the nonparametric Mann–Whitney test. A _P-_value of <0.05 was considered significant. RESULTS PRE-IRRADIATION OF THE MAMMARY GLAND
PROMOTES THE INVASION AND MIGRATION OF MOUSE MAMMARY CANCER CELLS To determine whether irradiation of the mammary gland provided a microenvironment conducive to the migration of cancer
cells, we first investigated the effect of radiation on the invasion capacity of D2A1 cells _in vitro_ by using invasion chambers. The BALB/c 3T3 fibroblasts were used to represent the
stroma and were plated in the lower compartment of the chamber before being irradiated at 0 or 5 Gy. Our results showed that irradiated fibroblasts acted as a chemoattractant, and increased
by 1.7-fold (_P=_0.003) the number of D2A1 cells that crossed the Matrigel layer (Figure 2A). Then, we assessed whether pre-irradiation of mice mammary gland had an effect on the migration
of D2A1 FUCCI-expressing cells by using an animal optical imager. At 1 week after their injection close to the nipple, cells within the nonirradiated control mammary glands were forming a
compact tumour at the site of implantation. In sharp contrast, in the pre-irradiated mammary glands, the D2A1 FUCCI-expressing cells had migrated away from the implantation site and were
forming tumours adopting an elongated shape. The migration distance from the injection site to the front of the tumour was increased by 1.8-fold (_P=_0.0095) in the pre-irradiated mammary
gland compared with the control nonirradiated one in the same animal (Figure 2B and C). Tumour volumes in the pre-irradiated mammary glands were also smaller (Figure 2D). This indicates that
radiation favours the migration and invasion of cancer cells that occurred at the expense of tumour growth. The experiment was repeated in an independent group of mice, for whom none of the
mammary glands had been irradiated. The distance of D2A1 FUCCI-expressing cell migration and growth within the mammary glands of these sham-treated mice was equivalent to those measured in
the nonirradiated mammary glands of mice whose opposite mammary gland had been pre-irradiated. These results ruled out the possibility that systemic factors induced by radiation modified the
migration of cancer cells implanted in the nonirradiated mammary gland. This supports the model of using a mouse in which one mammary gland is irradiated whereas the contralateral
nonirradiated gland acts as a control, thus avoiding interanimal variations. EFFECT OF RADIATION ON CELL CYCLE DISTRIBUTION Using the animal optical imager, only red fluorescence emitted by
the D2A1 FUCCI-expressing cells was observed in both sides of the mammary gland. This suggested that either cancer cells were concentrated in the G1 phase or the green fluorescence was
attenuated by tissues (Hillman et al, 2011). Therefore, histological analyses were performed on frozen tumour sections that revealed a high number of red and green cells. This result
supports the hypothesis that green fluorescence was attenuated by tissues. The tumour sections were then used to assess the effect of pre-irradiation of the mammary gland on the
proliferation of tumour cells by quantifying cells at the G1 phase (red fluorescence) and those in S/G2/M phases (green fluorescence). Radiation increased by 26% ratio of red-to-green cells
(_P_=0.0356) compared with the control tumours (Figure 2E and F). The correlation between the decrease of proliferating cells (green) and the stimulation of cancer cell migration supports
that pre-irradiation of the mammary gland promotes the migration of cancer cells while reducing the proliferation rate of tumour cells. PRE-IRRADIATION OF HEALTHY MAMMARY GLAND PROMOTES LUNG
METASTASES To assess whether the stimulation of cancer cell migration induced in the pre-irradiated mammary gland affected the development of metastases, the number of lung metastases was
quantified by optical imaging 21 days after the implantation of the D2A1 FUCCI-expressing cells. In the sham group, none of the mammary glands were irradiated before implantation of the D2A1
FUCCI-expressing cells on both sides. Although few metastases were observed in the lungs of sham-irradiated mice, the number of metastases in pre-irradiated animals increased by 2.4-fold
(_P_=0.0281; Figure 3A and B). Confirming the presence of metastases, frozen sections of lungs observed under fluorescence microscopy revealed strong red and green fluorescence signals
emitted by the metastases (Figure 3C). The ratio of red-to-green cells in the lung metastases was not affected irrespective of whether the D2A1 cells were implanted in either the
pre-irradiated or nonirradiated animals (results not shown). Supporting the hypothesis that irradiation of the mammary gland did not affect the proliferation rate of metastatic cells, the
diameters of pulmonary metastases in irradiated and nonirradiated animals were not significantly different (Figure 3D). Pre-irradiation of the mammary gland increased the number of lung
metastases but did not affect the metastatic cell proliferation rate. MECHANISMS INVOLVED IN RADIATION ENHANCEMENT OF PULMONARY METASTASES We first hypothesised that the higher number of
pulmonary metastases was caused by an increase of CTCs. The CTCs were easily distinguishable in blood samples and were quantified by fluorescence microscopy on days 4 and 7 after the
implantation of the D2A1 FUCCI-expressing cells in the mammary glands. Mice subjected to mammary gland pre-irradiation showed a two-fold increase in CTC on days 4 (_P_<0.0001) and 7
(_P_=0.0001) (Figure 4A). We next verified whether pre-irradiation of the mammary gland might have released systemic factors that would favour the extravasation of circulating cancer cells
to the lungs. This was assessed by directly injecting the D2A1 FUCCI-expressing cells (106) via the tail vein of mice with a pre-irradiated mammary gland, which were compared with a second
group of nonirradiated mice. The animals were killed 9 days later and their lungs removed to quantify the metastatic area by optical imaging. The number of lung metastases was not
significantly different between the sham and pre-irradiated groups, thus supporting the fact that the nesting of cancer cells in the lungs was not favoured by the pre-irradiation of a
mammary gland (Figure 4B). ASSESSMENT OF PROMIGRATORY AND INFLAMMATORY FACTORS To characterise these adverse effects of radiation, promigratory and inflammatory factors were quantified in
pre-irradiated mammary glands. As proteases are known to favour migration and invasion of cancer cells, the activity and/or levels of MMP-2 and MMP-9 were first determined by zymography.
Surprisingly, no radiation enhancement was observed with both MMPs in the mammary glands that were either implanted with D2A1 tumour or free of D2A1 tumours (Figure 5A and B), as analysed by
zymography. These results were validated by IHC analyses as heterogeneous increase of MMP-2/-9 could be missed when the analysis is done in the whole mammary glands by zymography. The IHC
results confirmed that MMP-2 expression was not increased in irradiated and nonirradiated mammary glands free of D2A1 tumour (Figure 5CI and CII). Similar levels of MMP-2 (Figure 5C and III
and C-IV) and MMP-9 (Figure 5CV and CVI) were also obtained in tumours implanted in irradiated or nonirradiated mammary glands. The MMP-2 was specifically localised in tumour periphery with
almost no expression in the tumour core. The MMP-9 was moderately expressed everywhere in mammary tumours but homogeneously. Likewise, the expression of MT1-MMP, an activator of these
proteases, was not stimulated by radiation (Figure 5D). We then characterised several induced inflammatory molecules in irradiated mammary glands. The relative expression of IL-6 was
significantly increased (_P_=0.0091), but not that of IL-1_β_, TGF-_β_1 or TNF-_α_, as measured by qPCR at 6 h post irradiation. Regarding the pathway of biosynthesis of PGE2 and PGD2, a
higher expression of COX-2 was found (_P_=0.0039), whereas a modest but nonsignificant increase of PLA2 expression was also observed. Interestingly, 15-PGDH expression, which metabolises
PGE2, was reduced (Figure 5D). The levels of prostaglandins PGE2 and PGD2, at different times post irradiation, were quantified by LC-MS/MS. A small increase, only for PGE2, was observed at
4 and 7 h post irradiation (Figure 5E). DISCUSSION Primary breast tumours are frequently removed by conservative surgery. However, 39–63% of patients display malignant microfoci scattered
throughout their breast (Holland et al, 1985). Therefore, protocols of radiotherapy include the whole breast and frequently a portion of the chest to include the axillary and supraclavicular
lymph nodes. Consequently, a large volume of healthy tissue receives a significant radiation dose causing inflammation (Rodemann and Blaese, 2007). The importance of the microenvironment in
tumour progression is becoming increasingly accepted (Goldberg and Schwertfeger, 2010). As inflammation can be associated with the promotion of metastases, it is important to determine
whether radiation-induced inflammation in healthy breast tissue could stimulate the migration of cancer cells and ultimately favour the formation of metastases. An enhancement of cancer cell
invasion after irradiation has been reported for pancreatic cancer cells (Qian et al, 2002), glioma cells (Wild-Bode et al, 2001; Park et al, 2006), melanoma cells (Rofstad et al, 2004;
Kaliski et al, 2005), rectal carcinoma cells (Speake et al, 2005) and colon carcinoma cells (Wang et al, 2000). These studies were designed to measure the invasiveness of irradiated cancer
cells that survived after radiation treatment. The present study was designed to investigate whether irradiation of the BALB/c mouse mammary gland could stimulate the migration of mouse
mammary cancer cells and the development of lung metastases. To test our hypothesis, mice mammary glands were pre-irradiated before implantation of the D2A1 mouse mammary cancer cells. This
protocol eliminated confounding effects such as the selection of cancer cells more likely to migrate, which could occur by irradiating the tumour and the mammary gland at the same time.
Following irradiation of the mammary gland, a substantial stimulation of D2A1 cell migration occurred at the expense of the growth of the primary tumours, which were smaller and more
elongated compared with the tumours implanted in nonirradiated mammary glands. A similar enhancement was measured _in vitro_ using invasion chambers in which irradiated fibroblasts
stimulated the invasiveness of nonirradiated D2A1 cells through a layer of Matrigel. The radiation enhancement of cancer cell migration was a local effect limited to the pre-irradiated
mammary gland. Indeed, migration of the D2A1 FUCCI-expressing cells in the opposite nonirradiated mammary gland was similar to the migration found in animals who did not have any of their
mammary glands irradiated. Therefore, irradiation did not seem to release pro-migratory cytokines into the circulation that would favour the migration of cancer cells in nonirradiated
tissues. The ability of an irradiated tissue to favour migration of cancer cells at the expense of growth of the primary tumour was previously reported in a glioblastoma rat model (Desmarais
et al, 2012). Brain irradiation before implantation of F98 glioma cells reduced the growth of the primary tumour and favoured the infiltration of cancer cells that migrated a longer
distance from the edges of the primary tumour. Notably, this switch from a proliferation to infiltration phenotype of the F98 cells reduced the mean survival time of the animals. The
emergence of a migratory phenotype is believed to be the consequence of acquired mutations in cancer cells. However, in our model, stimulation of the migratory phenotype was observed without
irradiating the cancer cells. These results led us to propose an alternative explanation based on pro-migratory molecules that trigger the transition from a proliferative phenotype to an
invasive one. A mutation-based hypothesis alone cannot explain the metastatic progression of all tumours. For example, a mutation-based hypothesis fails to explain the short time to
recurrence of glioblastoma multiforme (GBM) after tumour resection (Hatzikirou et al, 2010). Giese et al (2003) have reported that cell migration and proliferation are mutually exclusive
processes for glioma cells. In their model, glioma cells proliferated only when they did not move. It turns out that the proliferation and migration of tumour cells are mutually exclusive
phenotype. This mechanism, known as the migration/proliferation dichotomy (or the ‘Go or Grow’ mechanism), is also supported by experimental evidence showing the lower proliferation rate of
migratory cells in comparison with the tumour core (Giese et al, 2003). In our study with pre-irradiated mammary glands, we implanted D2A1 cells that expressed the FUCCI cell cycle marker.
This tool allowed us to confirm a transition to the G1 phase and a depletion of the S/G2/M phases in the tumour cells implanted in pre-irradiated mammary glands, thus supporting a transition
from the proliferative to migratory phenotypes. Cell migration is a coordinated process, and it is likely that changes in the expression of several genes are required for cancer cells to
become mobile. Carcinomas can undergo an epithelial-to-mesenchymal transition (EMT) and then move through a matrix-filled space by using proteases (Nabeshima et al, 2002). Transforming
growth factor-_β_1 can increase the migration of cancer cells by inducing an EMT (Romagnoli et al, 2012). In the pre-irradiated mammary glands of BALB/c mice, TGF-_β_1 gene expression was
not increased. A similar result was reported by Barcellos-Hoff et al (1994) who described an increase of the activation of latent TGF-_β_1 by radiation rather than an elevation of gene
expression (Barcellos-Hoff, 1993). Moreover, inflammation is a very dynamic process. Our specific time point may have missed TGF-_β_1 gene expression, as well as any other inflammatory
mediators that were not reported to be increased by radiation in this study. However, the majority of solid tumours do not undergo an EMT (Sahai, 2005). These cancer cells migrate by
adopting an amoebid style of movement that does not require proteases because the cells are able to squeeze through gaps in the extracellular matrix (ECM) (Sahai, 2005). By using _in vivo_
videomicroscopy, it was previously reported that D2A1 cells in mouse liver can squeeze through hepatocytes (Morris et al, 1994). Levels of the MMP-2 and MMP-9 proteases were not
significantly increased in the pre-irradiated mammary gland or in the D2A1 tumours. Nevertheless, MMP-2 was probably helping the migration of cancer cells as a high expression of this
protease was found in the mammary glands. Supporting a potential role of MMP-2 in tumour progression, the IHC analyses demonstrated that MMP-2 was expressed exclusively in tumour periphery.
We cannot also rule out the role for MMP-9 and the MMP activator MT1-MMP in radiation-induced migration because it was reported that radiotherapy increased by 2- to 18-fold the plasma level
of MMP-9 in women with breast cancer (Riekki et al, 2000). We propose, in our animal model, that the increase of D2A1 cancer cell migration would be associated with amoeboid-like movement
and MMP-2. Therefore, whether MMP inhibitors could have a beneficial role in the prevention of the radiation enhancement of metastasis remains to be assessed. Extravasation of circulating
cancer cells to organs is in part reliant on the expression of adhesion molecules like the intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on the
surface of endothelial cells. Their expression can be stimulated by IL-1_β_ and TNF-_α_, but not by IL-6 (ten Kate et al, 2006). In our mouse model, only the expression of IL-6 was promoted
by radiation. The extravasation rate of circulating cancer cells did not seem to be affected by pre-irradiation of the mammary gland, as intravenous injection of D2A1 FUCCI-expressing cells
in the tail vein led to a similar number of lung metastases in the pre-irradiated and nonirradiated animals. However, the stimulation of lung metastases induced by radiation was associated
with an elevation of CTCs. The mechanisms responsible for this enhancement of CTC induced by pre-irradiation of the mammary gland are unclear. The increase in CTC in our model does not seem
to be attributed to a stimulation of angiogenesis within the mammary gland. Vascular endothelial growth factor (VEGFA) expression within the mammary gland, as assessed by qPCR (Supplementary
Figure 1A), was not enhanced 6 h after irradiation. The number of blood vessels did not increase either, which were quantified by immunohistochemistry with the CD31 endothelial cell marker
within the tumour-bearing pre-irradiated mammary gland (Supplementary Figure 1B and Supplementary Methods). However, whether pre-irradiation might, by increasing inflammatory cytokines,
promote vascular permeability or damage to the basement membrane within the mammary gland (thereby facilitating access of the cancer cells to the circulation) will require further
investigation. Cyclooxygenase-2 is a key enzyme in the inflammatory response that mainly produces PGE2. Notably, elevated expression of COX-2 in human breast cancer biopsies has been
associated with distant metastases and poor prognoses (Ranger et al, 2004; Zerkowski et al, 2007). Although COX-2 is known to be upregulated by radiation (Yang et al, 2011), its inhibition
was shown to decrease tumour growth, angiogenesis and metastasis in breast cancer mouse models (Chang et al, 2004; Greenhough et al, 2009; Tian and Schiemann, 2010). To counterbalance COX-2,
PGE2 is degraded by 15-PGDH. Interestingly, Wolf et al (2006) showed that a low level of 15-PGDH was found in highly metastatic breast carcinoma MDA-MB-231 cells and an upregulation of
15-PGDH significantly decreased their ability to form tumours in athymic mice. Our study supports such a role for COX-2 and PGE2 as a stimulation of PGE2 and a reduction of 15-PGDH were
concurrently associated with the promotion of cancer cell migration and lung metastases. We have also previously shown _in vitro_ that PGE2 enhanced breast cancer cell invasion, whereas
COX-2 inhibitor prevented radiation enhancement of breast cancer cell invasion (Paquette et al, 2011). Therefore, it would be interesting in future _in vivo_ studies to evaluate whether the
use of COX-2 inhibitors might represent an efficient way to prevent radiation-induced lung metastases. In conclusion, we have shown in the current study that pre-irradiation of the mammary
gland increased the migration of mouse mammary cancer cells, the quantity of circulating cancer cells and the number of lung metastases (Figure 6). These adverse effects were not due to
mutations induced by radiation in cancer cells, but rather to pro-migratory molecules induced in the microenvironment of irradiated mammary glands. On the other hand, we cannot exclude that
vascular and microenvironment changes occurring during tumour growth could also contribute to the migration of cancer cells after irradiation. In clinic, our results might suggest that the
efficiency of radiotherapy could be improved by preventing the stimulation of cancer cell migration induced by radiation. CHANGE HISTORY * _ 01 OCTOBER 2013 This paper was modified 12 months
after initial publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Balin-Gauthier D, Delord JP, Rochaix P, Mallard V, Thomas F, Hennebelle I,
Bugat R, Canal P, Allal C (2006) _In vivo_ and _in vitro_ antitumor activity of oxaliplatin in combination with cetuximab in human colorectal tumor cell lines expressing different level of
EGFR. _Cancer Chemother Pharmacol_ 57: 709–718. Article CAS Google Scholar * Barcellos-Hoff MH (1993) Radiation-induced transforming growth factor beta and subsequent extracellular matrix
reorganization in murine mammary gland. _Cancer Res_ 53: 3880–3886. CAS PubMed Google Scholar * Barcellos-Hoff MH (2010) Stromal mediation of radiation carcinogenesis. _J Mammary Gland
Biol Neoplasia_ 15: 381–387. Article Google Scholar * Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA (1994) Transforming growth factor-beta activation in irradiated murine mammary
gland. _J Clin Invest_ 93: 892–899. Article CAS Google Scholar * Barcellos-Hoff MH, Ravani SA (2000) Irradiated mammary gland stroma promotes the expression of tumorigenic potential by
unirradiated epithelial cells. _Cancer Res_ 60: 1254–1260. CAS PubMed Google Scholar * Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, Lane TF, Hla T (2004) Role of
prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breastcancer progression. _Proc Natl Acad Sci USA_ 101: 591–591-596. Article CAS Google Scholar * Clarke M,
Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y Early Breast Cancer Trialists'
Collaborative Group (EBCTCG) (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the
randomised trials. _Lancet_ 366: 2087–2106. Article CAS Google Scholar * Desmarais G, Fortin D, Bujold R, Wagner R, Mathieu D, Paquette B (2012) Infiltration of glioma cells in brain
parenchyma stimulated by radiation in the F98/Fischer rat model. _Int J Radiat Biol_ 88 (8): 565–574. Article CAS Google Scholar * Gallet P, Phulpin B, Merlin JL, Leroux A, Bravetti P,
Mecellem H, Tran N, Dolivet G (2011) Long-term alterations of cytokines and growth factors expression in irradiated tissues and relation with histological severity scoring. _PLoS One_ 6:
e29399. Article CAS Google Scholar * Giese A, Bjerkvig R, Berens ME, Westphal M (2003) Cost of migration: invasion of malignant gliomas and implications for treatment. _J Clin Oncol_ 21:
1624–1636. Article CAS Google Scholar * Goldberg JE, Schwertfeger KL (2010) Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. _Curr
Drug Targets_ 11: 1133–1146. Article CAS Google Scholar * Greenhough A, Smartt HJM, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaid A (2009) The COX-2/PGE 2 pathway: key roles in the
hallmarks of cancer and adaptation to the tumour microenvironment. _Carcinogenesis_ 30: 377–386. Article CAS Google Scholar * Hatzikirou H, Basanta D, Simon M, Schaller K, Deutsch A
(2010) ‘Go or Grow’: the key to the emergence of invasion in tumour progression? _Math Med Biol_ 29 (1): 49–65. Article Google Scholar * Hillman EM, Amoozegar CB, Wang T, McCaslin AF,
Bouchard MB, Mansfield J, Levenson RM (2011) _In vivo_ optical imaging and dynamic contrast methods for biomedical research. _Philos Transact A Math Phys Eng Sci_ 369: 4620–4643. Article
Google Scholar * Holland R, Veling SH, Mravunac M, Hendriks JH (1985) Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery.
_Cancer_ 56: 979–990. Article CAS Google Scholar * Kaliski A, Maggiorella L, Cengel KA, Mathe D, Rouffiac V, Opolon P, Lassau N, Bourhis J, Deutsch E (2005) Angiogenesis and tumor growth
inhibition by a matrix metalloproteinase inhibitor targeting radiation-induced invasion. _Mol Cancer Ther_ 4: 1717–1728. Article CAS Google Scholar * Lemay R, Archambault M, Tremblay L,
Bujold R, Lepage M, Paquette B (2011) Irradiation of normal mouse tissue increases the invasiveness of mammary cancer cells. _Int J Radiat Biol_ 87: 472–482. Article CAS Google Scholar *
Madani I, De Neve W, Mareel M (2008) Does ionizing radiation stimulate cancer invasion and metastasis? _Bull Cancer_ 95: 292–300. CAS PubMed Google Scholar * Mantovani A, Allavena P, Sica
A, Balkwill F (2008) Cancer-related inflammation. _Nature_ 454: 436–444. Article CAS Google Scholar * Morris VL, Koop S, MacDonald IC, Schmidt EE, Grattan M, Percy D, Chambers AF, Groom
AC (1994) Mammary carcinoma cell lines of high and low metastatic potential differ not in extravasation but in subsequent migration and growth. _Clin Exp Metastasis_ 12: 357–367. Article
CAS Google Scholar * Nabeshima K, Inoue T, Shimao Y, Sameshima T (2002) Matrix metalloproteinases in tumor invasion: role for cell migration. _Pathol Int_ 52: 255–264. Article CAS Google
Scholar * Paquette B, Therriault H, Desmarais G, Wagner R, Royer R, Bujold R (2011) Radiation-enhancement of MDA-MB-231 breast cancer cell invasion prevented by a cyclooxygenase-2
inhibitor. _Br J Cancer_ 105: 534–541. Article CAS Google Scholar * Park CM, Park MJ, Kwak HJ, Lee HC, Kim MS, Lee SH, Park IC, Rhee CH, Hong SI (2006) Ionizing radiation enhances matrix
metalloproteinase-2 secretion and invasion of glioma cells through Src/epidermal growth factor receptor-mediated p38/Akt and phosphatidylinositol 3-kinase/Akt signaling pathways. _Cancer
Res_ 66: 8511–8519. Article CAS Google Scholar * Qian LW, Mizumoto K, Urashima T, Nagai E, Maehara N, Sato N, Nakajima M, Tanaka M (2002) Radiation-induced increase in invasive potential
of human pancreatic cancer cells and its blockade by a matrix metalloproteinase inhibitor, CGS27023. _Clin Cancer Res_ 8: 1223–1227. CAS PubMed Google Scholar * Rak JW, McEachern D,
Miller FR (1992) Sequential alteration of peanut agglutinin binding-glycoprotein expression during progression of murine mammary neoplasia. _Br J Cancer_ 65: 641–648. Article CAS Google
Scholar * Ranger GS, Thomas V, Jewell A, Mokbel K (2004) Elevated cyclooxygenase-2 expression correlates with distant metastases in breast cancer. _Anticancer Res_ 24: 2349–2351. CAS
PubMed Google Scholar * Riekki R, Jukkola A, Sassi ML, Hoyhtya M, Kallioinen M, Risteli J, Oikarinen A (2000) Modulation of skin collagen metabolism by irradiation: collagen synthesis is
increased in irradiated human skin. _Br J Dermatol_ 142: 874–880. Article CAS Google Scholar * Rodemann HP, Blaese MA (2007) Responses of normal cells to ionizing radiation. _Semin Radiat
Oncol_ 17: 81–88. Article Google Scholar * Rofstad EK, Mathiesen B, Galappathi K (2004) Increased metastatic dissemination in human melanoma xenografts after subcurative radiation
treatment: radiation-induced increase in fraction of hypoxic cells and hypoxia-induced up-regulation of urokinase-type plasminogen activator receptor. _Cancer Res_ 64: 13–18. Article CAS
Google Scholar * Romagnoli M, Belguise K, Yu Z, Wang X, Landesman-Bollag E, Seldin DC, Chalbos D, Barille-Nion S, Jezequel P, Seldin ML, Sonenshein GE (2012) Epithelial-to-mesenchymal
transition induced by TGF-beta1 is mediated by Blimp-1-dependent repression of BMP-5. _Cancer Res_ 72: 6268–6278. Article CAS Google Scholar * Sahai E (2005) Mechanisms of cancer cell
invasion. _Curr Opin Genet Dev_ 15: 87–96. Article CAS Google Scholar * Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H,
Imamura T, Ogawa M, Masai H, Miyawaki A (2008) Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. _Cell_ 132: 487–498. Article CAS Google Scholar * Speake WJ,
Dean RA, Kumar A, Morris TM, Scholefield JH, Watson SA (2005) Radiation induced MMP expression from rectal cancer is short lived but contributes to _in vitro_ invasion. _Eur J Surg Oncol_
31: 869–874. Article CAS Google Scholar * ten Kate M, Hofland LJ, van Koetsveld PM, Jeekel J, van Eijck CH (2006) Pro-inflammatory cytokines affect pancreatic carcinoma cell. Endothelial
cell interactions. _JOP_ 7: 454–464. PubMed Google Scholar * Tian M, Schiemann WP (2010) PGE2 receptor EP2 mediates the antagonistic effect of COX-2 on TGF-beta signaling during mammary
tumorigenesis. _FASEB J_ 24: 1105–1116. Article CAS Google Scholar * Wang JL, Sun Y, Wu S (2000) Gamma-irradiation induces matrix metalloproteinase II expression in a p53-dependent
manner. _Mol Carcinog_ 27: 252–258. Article CAS Google Scholar * Wild-Bode C, Weller M, Wick W (2001) Molecular determinants of glioma cell migration and invasion. _J Neurosurg_ 94:
978–984. Article CAS Google Scholar * Wolf I, O'Kelly J, Rubinek T, Tong M, Nguyen A, Lin BT, Tai HH, Karlan BY, Koeffler HP (2006) 15-Hydroxyprostaglandin dehydrogenase is a tumor
suppressor of human breast cancer. _Cancer Res_ 66: 7818–7823. Article CAS Google Scholar * Wu P, van Overbeek M, Rooney S, de Lange T (2010) Apollo contributes to G overhang maintenance
and protects leading-end telomeres. _Mol Cell_ 39: 606–617. Article CAS Google Scholar * Yang HJ, Youn H, Seong KM, Yun YJ, Kim W, Kim YH, Lee JY, Kim CS, Jin YW, Youn B (2011)
Psoralidin, a dual inhibitor of COX-2 and 5-LOX, regulates ionizing radiation (IR)-induced pulmonary inflammation. _Biochem Pharmacol_ 82: 524–534. Article CAS Google Scholar * Yang P,
Felix E, Madden T, Fischer SM, Newman RA (2002) Quantitative high-performance liquid chromatography/electrospray ionization tandem mass spectrometric analysis of 2- and 3-series
prostaglandins in cultured tumor cells. _Anal Biochem_ 308: 168–177. Article CAS Google Scholar * Zerkowski MP, Camp RL, Burtness BA, Rimm DL, Chung GG (2007) Quantitative analysis of
breast cancer tissue microarrays shows high cox-2 expression is associated with poor outcome. _Cancer Invest_ 25: 19–26. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS
BP, RB and CS are members of the Fonds de la Recherche en Santé du Québec (FRSQ)-funded Centre de recherche clinique Étienne-LeBel. CS is a FRSQ scholar and is also funded by a researcher of
the Canadian Foundation for Innovation. We thank Dr Ann Chambers for generously providing the D2A1mouse mammary cancer cells. We thank Dr Asako Sakaue-Sawano for kindly providing the
lentiviral vectors coding for the FUCCI genes. The medical physicists, Patrick Delage and Vincent-Hubert Tremblay, are thanked for their very helpful dosimetry calculations for mice
irradiation. We also thank Dr Chang Shu Wang for the BED calculations for the animals. Finally, special thanks to Chantal Mitterer for all of the implementation techniques for the _in vivo_
imaging. This research project was supported by the Canadian Institutes of Health Research (Grant 184671). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Nuclear Medicine and
Radiobiology, Centre for Research in Radiotherapy, Université de Sherbrooke, 3001, 12e Avenue Nord, Sherbrooke, J1H 5N4, Québec, Canada G Bouchard, G Bouvette, H Therriault, R Bujold & B
Paquette * Service of Radiation Oncology, Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, Québec, Canada R Bujold * Department of Anatomy and Cellular Biology, Faculty of
Medicine and Health Sciences, Université de Sherbrooke, Sherbrooke, Québec, Canada C Saucier Authors * G Bouchard View author publications You can also search for this author inPubMed Google
Scholar * G Bouvette View author publications You can also search for this author inPubMed Google Scholar * H Therriault View author publications You can also search for this author
inPubMed Google Scholar * R Bujold View author publications You can also search for this author inPubMed Google Scholar * C Saucier View author publications You can also search for this
author inPubMed Google Scholar * B Paquette View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to B Paquette. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION This work is published under the standard license to publish agreement. After 12 months
the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. Supplementary Information
accompanies this paper on British Journal of Cancer website SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 (JPG 505 KB) SUPPLEMENTARY FIGURE LEGENDS (DOC 26 KB) SUPPLEMENTARY TABLE 1 (DOC
37 KB) SUPPLEMENTARY INFORMATION (DOC 26 KB) RIGHTS AND PERMISSIONS From twelve months after its original publication, this work is licensed under the Creative Commons
Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Bouchard, G., Bouvette, G., Therriault, H. _et al._ Pre-irradiation of mouse mammary gland stimulates cancer cell migration and development of lung metastases. _Br
J Cancer_ 109, 1829–1838 (2013). https://doi.org/10.1038/bjc.2013.502 Download citation * Received: 03 April 2013 * Revised: 31 July 2013 * Accepted: 02 August 2013 * Published: 03 September
2013 * Issue Date: 01 October 2013 * DOI: https://doi.org/10.1038/bjc.2013.502 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * breast cancer *
irradiation * mammary gland * metastasis * cancer cell migration
