A systematic review and meta-analysis of the association between childhood infections and the risk of childhood acute lymphoblastic leukaemia
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT BACKGROUND: To determine whether childhood infections were associated with the development of childhood acute lymphoblastic leukaemia (ALL). METHODS: We included studies that
assessed any infection in childhood prior to the diagnosis of ALL in children aged 0–19 years compared to children without cancer. The primary analysis synthesised any infection against the
odds of ALL, and secondary analyses assessed the frequency, severity, timing of infections, and specific infectious agents against the odds of ALL. Subgroup analyses by data source were
investigated. RESULTS: In our primary analysis of 12 496 children with ALL and 2 356 288 children without ALL from 38 studies, we found that any infection was not associated with ALL (odds
ratio (OR)=1.10, 95% CI: 0.95–1.28). Among studies with laboratory-confirmed infections, the presence of infections increased the odds of ALL by 2.4-fold (OR=2.42, 95% CI: 1.54–3.82).
Frequency, severity, and timing of infection were not associated with ALL. CONCLUSIONS: The hypothesis put forward by Greaves and others about an infectious aetiology are neither confirmed
nor refuted and the overall evidence remains inadequate for good judgement. The qualitative difference in the subgroup effects require further study, and future research will need to address
the challenges in measuring infectious exposures. SIMILAR CONTENT BEING VIEWED BY OTHERS PREVALENCE OF HEMATOLOGICAL MALIGNANCIES IN AFRICA: A SYSTEMATIC REVIEW AND META-ANALYSIS Article
Open access 19 March 2025 NUMBER OF SIBLINGS AND SURVIVAL FROM CHILDHOOD LEUKAEMIA: A NATIONAL REGISTER-BASED COHORT STUDY FROM SWEDEN Article 14 April 2021 PROGRESS AGAINST CHILDHOOD AND
ADOLESCENT ACUTE LYMPHOBLASTIC LEUKAEMIA IN THE NETHERLANDS, 1990–2015 Article Open access 21 August 2020 MAIN The aetiology of childhood acute lymphoblastic leukaemia (ALL) is largely
unknown, and likely arises from interactions between exogenous and/or endogenous exposures, genetic susceptibility, and chance. Genetic causes of ALL account for a small proportion of cases,
and while the disease is usually initiated in utero, other promotional exposures are probably necessary for disease emergence (Greaves et al, 2003). There are two key hypotheses on
infections and the development of ALL. Kinlen proposed the ‘population mixing’ hypothesis to describe the observed increased rates of childhood ALL following an influx of migrants into rural
areas (Kinlen, 1988, 2012). Briefly, the mixing of rural, isolated individuals with the influx of mostly urban individuals into a rural area would create a localised epidemic of an
underlying infection due to the increased level of contact between susceptible and infected individuals that may produce the rare response of ALL. Studies from Kinlen and others have found
evidence to support the hypothesis (Kinlen, 1988, 2006, 2012; Alexander et al, 1998; Kinlen and Doll, 2004). The hypothesis suggests a direct pathological role of a specific infection,
presumed to be viral, in the development of ALL and that a protective effect may be acquired from previous exposure. Currently, there is limited molecular evidence that implicates a specific
infection (Martin-Lorenzo et al, 2015; da Conceicao Nunes et al, 2016). Greaves’ ‘delayed infection’ hypothesis for childhood ALL suggests a two-hit model that emphasises the timing of
exposure and the child’s immune system (Greaves, 1997, 2006). The first hit occurs in utero through one’s genetic makeup that produces a pre-leukaemic clone. In a small number of
pre-leukaemia carriers, it is the absence of exposure to infections in early life, and a postnatal secondary genetic event caused by a delayed, stress-induced infection (second hit) on the
developing, ‘unprepared’ immune system that may increase the risk of childhood ALL. Although the mechanisms differ, both hypotheses suggest that ALL is a rare response to one or more common
infections acquired through personal contact. The difficulties in measuring exposure to infectious agents and subsequent responses make it challenging to directly test the hypotheses,
especially since no specific leukaemogenic agent has been identified. Several previous epidemiological studies have used a history of infections as an indicator for early exposure to
infections. Establishing the timing of the infections is critical to testing the hypotheses; however, birth cohort studies are not feasible given the rarity of childhood ALL. Thus, most
studies used a case–control design and interviews to measure infections. Assessing a history of infections through interviews can be problematic due to the potential for recall bias and
misclassification of children who had asymptomatic infections (Simpson et al, 2007). Other methods for measuring infections such as using administrative data overcome these limitations, but
may lack information on important confounders. Other than narrative summaries (McNally and Eden, 2004; Buffler et al, 2005; Ma et al, 2009; Maia Rda and Wunsch Filho, 2013), no study has
attempted to synthesise and quantitatively pool studies examining the relationship using a history of infections, or tried to explain the differences between the studies. The aim of this
systematic review and meta-analysis was to assess the relationship between childhood infections, and the development childhood ALL by summarising the findings for an overall measure of
infections, the frequency, severity, timing of infections, and examining specific infectious agents and syndromes. MATERIALS AND METHODS The Meta-analysis of Observational Studies in
Epidemiology (MOOSE) was developed as a guideline for the reporting of meta-analyses of observational studies in epidemiology and was used for the current study (Stroup et al, 2000). DATA
SOURCES AND SEARCHES We performed electronic searches from inception to 21 February 2017 in Ovid MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, Web of Science (Science
Citation Index Expanded, Social Sciences Citation Index, Conference Proceedings Citation Index for both Science and Social Science & Humanities), and Scopus. Supplementary Table 1 shows
the search strategies used. Text words used included _acute lymphoblastic leukaemia_, _acute leukaemia_, _infection_, _virus_, and _bacteria_. We limited the search to subjects 0–19 years
old, and did not restrict the search by language. References of the included studies were searched, and the first four pages of a Google search using the same keywords were used to search
for grey literature. STUDY SELECTION We defined the inclusion and exclusion criteria a priori as studies of any design excluding editorials, reviews, and case reports. Studies were included
if: (1) the primary exposure of interest included a prior history of any infection before the diagnosis of childhood ALL; (2) the primary outcome of interest was defined as clinically
diagnosed ALL in children aged ⩽19 years; (3) comparisons were made against a control or comparison group; and (4) testing samples must have been collected and assessed prior to treatment,
if laboratory investigations were used to determine past infections. Infections must have been reported by the parent or guardian, or obtained through other data sources such as medical
records. We excluded studies based on the following order: (1) definition for infections was not at the individual level, for example, at an ecological level that examines infections
aggregated for a region; (2) definition for infections that examined population mixing; (3) infections were not explicitly infections during childhood (e.g., infections during pregnancy);
(4) outcomes was not childhood ALL in children aged ⩽19 years; (5) absence of a comparison group; (6) it was a review article; and (7) duplicate publication with the same study population.
When more than one publication from a study was available, the most recent version, or the version with the exposure or outcome of interest that was closest to the objectives of this review
was included. Studies were not restricted by publication status, and relevant studies in other languages were translated. Two reviewers (JH and CT) independently evaluated the titles and
abstracts of publications identified by the search strategy, and any publication thought to be potentially relevant by either reviewer was retrieved in full. Final inclusion of studies in
the systematic review was determined by agreement of both reviewers. Agreement between reviewers was evaluated using the kappa statistic (_κ_). Strength of agreement was defined as slight
(_κ_=0.00–0.20), fair (_κ_=0.21–0.40), moderate (_κ_=0.41–0.60), substantial (_κ_=0.61–0.80), or almost perfect (_κ_=0.81–1.00) (Landis and Koch, 1977). DATA EXTRACTION AND QUALITY
ASSESSMENT Data extraction was conducted in duplicate (JH and CT) using a standard form, which collected information on: the primary exposure of ‘common infections’, defined as any infection
occurring from birth to the diagnosis of ALL; secondary exposures of infection frequency, severity of infections; and study design, region, publication era, and source of controls. In
studies that used laboratory investigations for identification of infectious agents, we extracted IgG antibody estimates to represent past infections, and if that was not available, the
polymerase chain reactions (PCR) method was extracted to assess for the presence of the agent. We extracted infections occurring in the first year of life or similar time windows in cases
with multiple time windows, as we felt this best represented early exposure to infections. We extracted infection frequency levels for common infections, and defined severity based on
admission to hospital. The adjusted models that incorporated the most confounders for our primary outcome ALL were extracted. Authors were contacted for further information regarding results
that were not presented. Five authors were contacted (Nishi and Miyake, 1989; Schlehofer et al, 1996; Neglia et al, 2000; Rosenbaum et al, 2005; MacArthur et al, 2008), and three responded
with no additional information (Nishi and Miyake, 1989; Neglia et al, 2000; Rosenbaum et al, 2005). Study quality was assessed using the Meta Quality Appraisal Tool (MetaQAT) (Rosella et al,
2015) and the Critical Appraisal Skills Programme (CASP) for case–control (Programme CAS, 2014a), and cohort studies (Programme CAS, 2014b). Two reviewers (JH and CT) assessed each study.
For case–control studies, we considered CASP scores of 1–3, 4–6, and 7–9 to be high, moderate, and low-risk of bias, respectively; for cohort studies, we considered CASP scores of 1–4, 5–8,
and 9–11 to be high, moderate and low-risk of bias, respectively. DATA SYNTHESIS AND ANALYSIS METHODS Our analysis combined data at the study level. Our primary analysis sought to assess
exposure to common infections _vs_ no common infections (referent group) on the risk of developing ALL, relying on each study’s definition. The most frequent infection was used when studies
did not report a common infection variable. We used the adjusted odds ratio (OR) or rate ratio (RR) to calculate a pooled overall effect, and assumed OR and RR were equivalent due to the
rarity of the outcome (Greenland, 1987); ORs or RRs <1 suggest infections are protective against ALL. If a study presented multiple frequency categories, we used the lowest _vs_ the
highest category, a method commonly used in meta-analyses (Bae, 2016). The method described by Greenland was used to calculate the variance using the reported 95% confidence intervals (CI)
(Greenland, 1987). We calculated a crude OR for studies not reporting one, and to facilitate the calculation we added 0.5 to all cells if one of the four cells reported a zero (Gart and Nam,
1988). In secondary analyses, we used the different exposure levels of infection to compute a regression slope (Greenland and Longnecker, 1992). If an exposure level was defined using a
range, we used the midpoint of the range (e.g., 1–3 infections was assigned a frequency of 2), and if the level was ⩾4, we assigned a frequency of 4. For infection severity, a dichotomous
variable (yes _vs_ no) was used to determine the relationship with ALL. _Post hoc_ analyses examined the timing of infections in the first year of life compared to infections that occurred
after the first year of life, and putative infectious agents was conducted if ⩾3 studies reported the agent. As we anticipated heterogeneity between the studies, we used an inverse variance
weighted average, random-effects model where the Wald-type tests and confidence intervals were estimated under a normal distribution (DerSimonian and Laird, 1986). We investigated potential
sources of heterogeneity using subgroup analyses and mixed-effects meta-regression. To examine the association of study-level characteristics and infection effect, we fitted mixed-effects
meta-regression models to the natural logarithm of the OR. The natural logarithm of the OR was assumed to have a normal distribution, and a method-of-moments-based estimator to estimate
model variables. The mixed-effects model included fixed effects for the covariates, and a random intercept term was specified to model residual heterogeneity not accounted for by the
covariates. We corrected for multiple testing using a Bonferroni correction that divides the _P_-value by the number of tests (Lagakos, 2006). Because of methodological differences (Wiemels,
2012), we tested for interactions to assess the differences between studies that used administrative/medical records, self-reported, and laboratory investigation data (Altman and Bland,
2003). We stratified infections in the first year of life by self-reported data and administrative/medical records data. We explored clinical heterogeneity by conducting a subgroup analysis
limiting cases of ALL to B-cell precursor ALL (Wiemels, 2012). We also explored the extent to which region (North America, Europe, Asia, or other), publication era (⩽1999, 2000–2009, ⩾2010),
source of controls (general population, general practitioner list, or hospital controls), and risk of bias influenced the magnitude of the average effect estimate in the meta-analysis.
Publication bias was assessed by funnel plot and the Egger’s test (Egger et al, 1997; Peters et al, 2008). The meta-analysis was performed using the metafor package in R, version 3.3
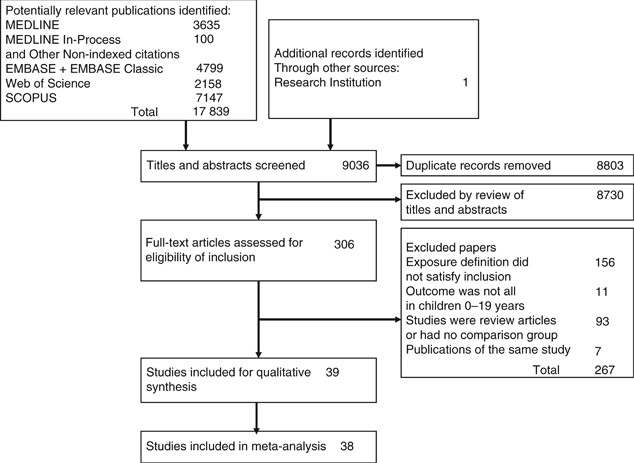
(Viechtbauer, 2010). RESULTS Titles and abstracts of 9445 records were reviewed and 314 full-text articles were retrieved (Figure 1). There were 39 studies that satisfied the inclusion
criteria (Till et al, 1979; van Steensel-Moll et al, 1986; Nishi and Miyake, 1989; Schlehofer et al, 1996; Dockerty et al, 1999; McKinney et al, 1999; Schuz et al, 1999; Neglia et al, 2000;
Mackenzie et al, 2001; Petridou et al, 2001; Chan et al, 2002; Perrillat et al, 2002; Salonen et al, 2002; Kerr et al, 2003; Canfield et al, 2004; Jourdan-Da Silva et al, 2004; Ma et al,
2005; Rosenbaum et al, 2005; Surico and Muggeo, 2005; Loutfy et al, 2006; Paltiel et al, 2006; Zaki et al, 2006; Roman et al, 2007; Cardwell et al, 2008; MacArthur et al, 2008; Flores-Lujano
et al, 2009; Tesse et al, 2009; Rudant et al, 2010; Zaki and Ashray, 2010; Mahjour et al, 2010; Ahmed et al, 2012; Chang et al, 2012; Vestergaard et al, 2013; Ibrahem et al, 2014; Ajrouche
et al, 2015; Lin et al, 2015; Rudant et al, 2015; da Conceicao Nunes et al, 2016; Ateyah et al, 2017), and of those, 38 were included in the meta-analysis. One study did not report
infections and the effect estimate could not be calculated (Paltiel et al, 2006). The reviewers had almost perfect agreement on the articles for inclusion (_κ_=0.85, 95% CI: 0.75, 0.95).
Characteristics of the included studies are presented in Table 1. The exposure definitions are presented in Supplementary Table 2. The reviewers had moderate agreement on the judgement of
the risk of bias for each study (_κ_=0.50, 95% CI: 0.28, 0.72). Thirteen studies were judged as being low-risk of bias, 7 as being moderate-risk of bias, and 19 as being high-risk of bias
(Supplementary Table 3a and b). We found evidence of publication bias (bias coefficient=1.19, 95% CI: 0.30, 2.08; Supplementary Figure 1). Our analysis included 12 496 children with ALL and
2 356 288 children without ALL. There was no association between infections and ALL, OR=1.10, 95% CI: 0.95, 1.28; _P_=0.187 (Figure 2). We observed considerable heterogeneity between the
studies (_I_2=76.5%; _Q_-statistic _P_<0.001). The trend analysis included 13 studies and we did not find frequency of infections to be associated with ALL (OR=1.00, 95% CI: 0.95, 1.05;
_P_=0.967). In the four studies that assessed the infection severity, the combined average effect of hospitalisations for infections was not associated with ALL (OR=1.22, 95% CI: 0.85, 1.75;
_P_=0.239). Infections that occurred in the first year of life was not associated with ALL (OR=0.99, 95% CI: 0.85, 1.16, _P_=0.920). Infections that occurred after the first year of life
suggested an association with ALL (OR=1.45, 95% CI: 0.71, 2.96, _P_=0.313), but did not differ compared to infections in the first year of life (interaction effect OR=0.69, 95% CI: 0.32,
1.43, _P_=0.314) (Supplementary Figure 2). Parvovirus B19 (OR=2.69, 95% CI: 1.16, 6.22, _P_=0.020) was found to be associated with ALL (Figure 2). No associations were observed for human
herpesvirus-6 (OR=0.89, 95% CI: 0.42, 1.87, _P_=0.752), however Epstein–Barr virus (OR=1.39, 95% CI: 0.83, 2.33, _P_=0.208), cytomegalovirus (OR=1.95, 95% CI: 0.64, 5.96, _P_=0.242),
influenza (OR=1.97, 95% CI: 0.97, 3.98, _P_=0.061), and herpes simplex virus (OR=2.04, 95% CI: 0.66, 6.23, _P_=0.214) showed a strong association to ALL, but lacked precision. Varicella,
rubella, mumps, measles, and pertussis were not associated with ALL (Supplementary Figure 3). SUBGROUP AND SENSITIVITY ANALYSES After applying the Bonferroni correction, the _P_-value to
indicate statistical significance for the additional analyses was <0.005. The data sources for the studies can be found in Table 1. Among the studies that used self-reported data, we
found no association between infections and ALL (OR=0.89, 95% CI: 0.79, 1.00, _P_=0.049; _I_2=50.5%). Among studies that used administrative/medical record data, we found no association
between infections and ALL (OR=1.00, 95% CI: 0.61, 1.63, _P_=0.994; _I_2=90.8%). Among studies that used laboratory data, we found infections to be associated with ALL (OR=2.42, 95% CI:
1.54, 3.82, _P_<0.001, _I_2=54.2%). The interaction effect showed no difference between self-reported and administrative/medical records data sources (OR=0.89, 95% CI: 0.54, 1.48,
_P_=0.656). Infections identified through laboratory data increased the risk of ALL compared to infections captured through self-reported data (interaction effect OR=2.73, 95% CI: 1.71,
4.36, _P_<0.001), but not administrative/medical records data sources (interaction effect OR=2.43, 95% CI: 1.24, 4.75, _P_=0.009). Among studies that used self-reported data, every
additional infection reduced the odds of ALL by 4% (OR=0.96, 95% CI: 0.94, 0.98; _P_<0.001), whereas among studies that used administrative/medical records data, every additional
infection increased the odds of ALL by 11% (OR=1.11, 95% CI: 1.07, 1.15; _P_<0.001). We found self-reported and administrative/medical records data sources qualitatively differed in the
frequency of infections (interaction effect OR=0.86, 95% CI: 0.83, 0.90, _P_<0.001). Severity of infections remained unchanged in studies with self-reported data (OR=1.51, 95% CI: 0.86,
2.65; _P_=0.158; _I_2=70.2%). Among self-reported studies, infections in the first year of life suggested a protective effect against ALL (OR=0.88, 95% CI: 0.80, 0.98, _P_=0.017). No
association was found between infections in the first year of life and ALL among administrative/medical records data (OR=0.93, 95% CI: 0.55, 1.56, _P_=0.775), and did not differ from
self-reported studies (interaction effect OR=0.95, 95% CI: 0.56, 1.62, _P_=0.862). The results from our primary analysis remained unchanged when we restricted the analysis to B-cell
precursor ALL or B-cell common ALL (OR=0.87, 95% CI: 0.77, 0.98, _P_=0.022). Meta-regression models that assessed study level characteristics included data source, region, publication era,
source of controls, and risk of bias. Data source and region accounted for the largest proportion of heterogeneity between the studies (_R_2=47.2%, see Supplementary Table 4). Stratification
by risk of bias indicated studies of low-risk of bias showed similar results to our main analysis (OR=0.92, 95% CI: 0.76, 1.10, _P_=0.349), whereas studies of moderate-to-high-risk of bias
suggested infections increased the risk of ALL (OR=1.45, 95% CI: 1.12–1.86, _P_=0.005). Compared to studies of moderate-to-high-risk of bias, studies of low-risk of bias were more likely to
suggest infections were protective against ALL (OR=0.63, 95% CI: 0.46, 0.87, _P_=0.004). DISCUSSION In this systematic review of 39 studies, we found no association between any common
infections, frequency, severity of infections, and timing of infections and childhood ALL. We did however, find a qualitative difference in our subgroup analyses; infections increased the
odds of developing ALL by 2.4-fold in studies with laboratory investigations. Further, infections identified through laboratory investigations increased the odds of ALL by 2.7-fold and
2.4-fold compared to infections identified through self-reported and administrative/medical records data, respectively. Among studies that used self-reported data, we found each additional
infection reduced the odds of ALL by 4%, and this differed significantly from studies that used administrative/medical records data that suggested each additional infection increased the
odds of ALL by 11%. The heterogeneity between the studies remained a challenge and could partly be explained by differences in the data sources. We failed to demonstrate an association in
our primary analysis, but found associations in our secondary and subgroup analyses by data source. There are three plausible explanations for the observed findings. First, the apparent
results may be a chance finding from multiple testing. Second, the ascertainment of infections from parental recall has been shown to under-report childhood infections and may be inaccurate
in both the timing and occurrence of infections, compared to medical records (McKinney et al, 1991; Simpson et al, 2007). Despite these potential issues, studies that confirmed the
self-reported infections with medical records for accuracy and completeness still found an inverse association (Dockerty et al, 1999; Ajrouche et al, 2015). Although studies that used
medical records were void of recall bias, they were often unable to include other important confounders, such as ethnicity, parental occupation, maternal age, birth weight, and parity
(Dockerty et al, 2001; Hjalgrim et al, 2004; Ma et al, 2005; Lim et al, 2014). Finally, the findings from the laboratory studies must be interpreted with caution due to the study quality,
and smaller sample sizes and larger effect sizes as shown by the asymmetry of the funnel plot. The mutational mechanisms of ALL point to three potential pathways: (1) anomalies in
lineage-specific factors (ETV6-RUNX1, IKZF1, and PAX5); (2) flaws in receptor protein tyrosine kinases and their down-stream pathways; and (3) epigenetic modifiers (Whitehead et al, 2016).
Recent developments in genome and mouse model studies may change our initial understanding of the aetiology of ALL as new studies have generated new hypotheses with respect to identifying
potential infectious candidates (Martin-Lorenzo et al, 2015; Swaminathan et al, 2015). The presence of parvovirus B19 IgG antibodies is associated with the presence of ETV6-RUNX1 (Ibrahem et
al, 2014), and is associated with certain class II HLA alleles that are risk factors for the development of childhood ALL. Furthermore, parvovirus B19 has certain characteristics similar to
other oncoviruses, that is, its DNA genome persists indefinitely in human tissues following acute infection, causing mild or no disease, and upregulates pro-inflammatory cytokines
associated with ALL onset (Kerr and Mattey, 2015). The results from the small laboratory studies will require confirmation in larger population studies. Since half of 15-year-old adolescents
have specific antiparvovirus B19 antibodies (Young and Brown, 2004), the measurement of the clinical syndromes caused by parvovirus B19 may be preferred to assess manifestations of the
pathogen. Parvovirus B19 infection may provide only a subset of an oncogenic hit in a multistep carcinogenesis process. The qualitative differences in our findings support the hypothesis of
an alternative pathway for ALL development. Recent qualitative reviews have attempted to explain the positive association between infections and ALL and suggested studies that used medical
records or administrative data may be capturing children with an earlier than expected altered immune system. These children may respond differently to infections, have a greater propensity
to seek medical care when infections are contracted, and/or have a stronger immune response (Wiemels, 2012; Whitehead et al, 2016). The sensitivity to infections may be due to a lack of
immunomodulation from lower levels of anti-inflammatory cytokine interleukin-10 in newborns who later go on to develop ALL (Chang et al, 2011). As in previous reviews, there continues to be
substantial heterogeneity among the studies; however, our review focuses on specific objectives and highlights the recent developments of the field (McNally and Eden, 2004; Buffler et al,
2005; Greaves, 2006; Ma et al, 2009; Maia Rda and Wunsch Filho, 2013). There are several limitations of this study. The heterogeneity between the studies in the definition of infections, the
time period to observe the infections, and the evidence of publication bias was a challenge. We decided to use any common infection as our main exposure variable in the primary analysis
because we felt it to be the most appropriate measure that reflects the hypotheses from Kinlen and Greaves (Kinlen, 1988; Greaves, 2006). The heterogeneity likely stems from the unknown
aetiology of ALL, and one that requires further research. The limitation with laboratory investigation studies is the inability to disentangle temporality. The presence of the infectious
agent was assessed after a diagnosis of ALL was made and it is unknown if the agent was present before or after the onset of ALL. It is unclear whether the infection occurred before the
onset of ALL, or if the potentially reduced immune function because of ALL contributed to the contraction of specific infections. Further, the laboratory studies were appraised as high-risk
of bias, often small, and may not be generalisable. Despite the differences in the risk of bias amongst the included studies, our conclusions were unchanged after we stratified the analysis
to the 13 studies with a low-risk of bias. Another limitation was the quality of reporting in the studies included in the review. Most studies clearly reported their findings, but studies
published earlier tended to have incomplete reporting. Costs and feasibility are the usual barriers to establishing new large pregnancy and birth cohorts (Riley and Duncan, 2016), research
groups have instead combined existing cohorts to study childhood cancers (Brown et al, 2007; Metayer et al, 2013) and other diseases (Larsen et al, 2013). The increased power may help to
identify high risk or vulnerable, and understudied populations. The next step should focus on the measurement of infections and infectious exposures. The use of linked administrative data
provides a large population for study with accurate information on the timing of physician diagnosed infections, frequency, and severity of infections as answers to these questions remain
elusive. Enhancing the administrative data with surveys to obtain other infectious exposures such as day-care attendance, breastfeeding, or by applying emerging technologies that detect and
quantify the pathogen burden with greater speed, accuracy, and simplicity (Caliendo et al, 2013) in a subset sample would improve the accuracy and strengthen the measurement of infections.
Day-care attendance has been found to increase the risk of exposure to infections, and has been used as a proxy for infections. A meta-analysis found day-care attendance reduced the risk of
childhood ALL (Urayama et al, 2010). Breastfeeding has been found to reduce the risk of ALL through its immunologically active components, antibodies, and other elements that influence the
development of the infant’s immune system (Kwan et al, 2004; Martin et al, 2005; Amitay and Keinan-Boker, 2015). The challenge will be to disentangle the mechanistic pathways of the
infectious aetiology hypothesis by combining different measurements of infectious exposures to determine the total, direct, and indirect effect of infections on the risk of developing
childhood ALL. An infectious aetiology of ALL is suggestive in our study; however, the challenges in measuring infections must be addressed. Parvovirus B19 as a putative causal infectious
agent for childhood ALL needs to be tested in larger cohorts and the rather substantial point estimates from influenza, cytomegalovirus, and herpes simplex virus warrant a follow-up in
larger studies. Whether children with ALL have a dysregulated immune function present at birth requires further investigation. Only one study conducted an exploratory assessment on a key
aspect of Greaves’ hypothesis, the timing of the infections in early life (Crouch et al, 2012). Our future research aims to provide further insight on the timing of infections and the risk
of developing childhood ALL. The use of administrative data or medical records with linked laboratory data would overcome the challenges facing studies that used self-reported and laboratory
investigation data, and would be ideal to evaluate the association between childhood ALL and the timing and frequency of infections. The review has highlighted knowledge gaps surrounding
the relationship between childhood ALL and severity of infections. The causal association of infections will need to be tested in conjunction with other identified risk factors to quantify
the direct and indirect interaction and mediated effect of infections on ALL risk. These will be critical research questions in discovering the causes of childhood ALL and will be the
foundation for future studies that can combine epidemiologic, genetic, and environmental factors. CHANGE HISTORY * _ 09 JANUARY 2018 This paper was modified 12 months after initial
publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Ahmed HG, Osman SI, Ashankyty IM (2012) Incidence of Epstein–Barr virus in pediatric leukaemia
in the Sudan. _Clin Lymphoma Myeloma Leuk_ 12 (2): 127–131. Article PubMed Google Scholar * Ajrouche R, Rudant J, Orsi L, Petit A, Baruchel A, Lambilliotte A, Gambart M, Michel G,
Bertrand Y, Ducassou S, Gandemer V, Paillard C, Saumet L, Blin N, Hemon D, Clavel J (2015) Childhood acute lymphoblastic leukaemia and indicators of early immune stimulation: The Estelle
study (SFCE). _Br J Cancer_ 112: 1017–1026. Article CAS PubMed PubMed Central Google Scholar * Alexander FE, Boyle P, Carli PM, Coebergh JW, Draper GJ, Ekbom A, Levi F, McKinney PA,
McWhirter W, Michaelis J, Peris-Bonet R, Petridou E, Pompe-Kirn V, Plisko I, Pukkala E, Rahu M, Storm H, Terracini B, Vatten L, Wray N (1998) Spatial clustering of childhood leukaemia:
summary results from the EUROCLUS project. _Br J Cancer_ 77 (5): 818–824. Article CAS PubMed PubMed Central Google Scholar * Altman DG, Bland JM (2003) Interaction revisited: the
difference between two estimates. _BMJ_ 326 (7382): 219. Article PubMed PubMed Central Google Scholar * Amitay EL, Keinan-Boker L (2015) Breastfeeding and childhood leukaemia incidence:
a meta-analysis and systematic review. _JAMA Pediatr_ 169 (6): e151025. Article PubMed Google Scholar * Ateyah ME, Hashem ME, Abdelsalam M (2017) Epstein–Barr virus and regulatory T cells
in Egyptian paediatric patients with acute B lymphoblastic leukaemia. _J Clin Pathol_ 70 (2): 120–125. Article CAS PubMed Google Scholar * Bae J-M (2016) Comparison of methods of
extracting information for meta-analysis of observational studies in nutritional epidemiology. _Epidemiol Health_ 38: e2016003. Article PubMed PubMed Central Google Scholar * Brown RC,
Dwyer T, Kasten C, Krotoski D, Li Z, Linet MS, Olsen J, Scheidt P, Winn DM (2007) Cohort Profile: The International Childhood Cancer Cohort Consortium (I4C). _Int J Epidemiol_ 36 (4):
724–730. Article PubMed Google Scholar * Buffler PA, Kwan ML, Reynolds P, Urayama KY (2005) Environmental and genetic risk factors for childhood leukaemia: appraising the evidence.
_Cancer Investig_ 23 (1): 60–75. Article CAS Google Scholar * Caliendo AM, Gilbert DN, Ginocchio CC, Hanson KE, May L, Quinn TC, Tenover FC, Alland D, Blaschke AJ, Bonomo RA, Carroll KC,
Ferraro MJ, Hirschhorn LR, Joseph WP, Karchmer T, MacIntyre AT, Reller LB, Jackson AF Infectious Diseases Society of America (2013) Better tests, better care: improved diagnostics for
infectious diseases. _Clin Infect Dis_ 57 (Suppl 3): S139–S170. Article PubMed Google Scholar * Canfield KN, Spector LG, Robison LL, Lazovich D, Roesler M, Olshan AF, Smith FO, Heerema
NA, Barnard DR, Blair CK, Ross JA (2004) Childhood and maternal infections and risk of acute leukaemia in children with Down syndrome: a report from the Children’s Oncology Group. _Br J
Cancer_ 91 (11): 1866–1872. Article CAS PubMed PubMed Central Google Scholar * Cardwell CR, McKinney PA, Patterson CC, Murray LJ (2008) Infections in early life and childhood leukaemia
risk: a UK case–control study of general practitioner records. _Br J Cancer_ 99 (9): 1529–1533. Article CAS PubMed PubMed Central Google Scholar * Chan LC, Lam TH, Lau YL, Li CK, Yuen
HL, Lee CW, Ha SY, Yuen PMP, Leung NK, Patheal SL, Greaves MF, Alexander FE (2002) Is the timing of exposure to infection a major determinant of acute lymphoblastic leukaemia in Hong Kong?
_Paediatr Perinat Epidemiol_ 16 (2): 154–165. Article PubMed Google Scholar * Chang JS, Tsai CR, Tsai YW, Wiemels JL (2012) Medically diagnosed infections and risk of childhood leukaemia:
a population-based case–control study. _Int J Epidemiol_ 41 (4): 1050–1059. Article PubMed Google Scholar * Chang JS, Zhou M, Buffler PA, Chokkalingam AP, Metayer C, Wiemels JL (2011)
Profound deficit of IL10 at birth in children who develop childhood acute lymphoblastic leukaemia. _Cancer Epidemiol Biomarkers Prev_ 20 (8): 1736–1740. Article CAS PubMed PubMed Central
Google Scholar * Crouch S, Lightfoot T, Simpson J, Smith A, Ansell P, Roman E (2012) Infectious illness in children subsequently diagnosed with acute lymphoblastic leukaemia: modeling the
trends from birth to diagnosis. _Am J Epidemiol_ 176 (5): 402–408. Article PubMed Google Scholar * da Conceicao Nunes J, de Araujo GV, Viana MT, Sarinho ES (2016) Association of atopic
diseases and parvovirus B19 with acute lymphoblastic leukaemia in childhood and adolescence in the northeast of Brazil. _Int J Clin Oncol_ 21 (5): 989–995. Article PubMed CAS Google
Scholar * DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. _Control Clin Trials_ 7 (3): 177–188. Article CAS PubMed Google Scholar * Dockerty JD, Draper G, Vincent T,
Rowan SD, Bunch KJ (2001) Case–control study of parental age, parity and socioeconomic level in relation to childhood cancers. _Int J Epidemiol_ 30 (6): 1428–1437. Article CAS PubMed
Google Scholar * Dockerty JD, Skegg DC, Elwood JM, Herbison GP, Becroft DM, Lewis ME (1999) Infections, vaccinations, and the risk of childhood leukaemia. _Br J Cancer_ 80 (9): 1483–1489.
Article CAS PubMed PubMed Central Google Scholar * Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. _BMJ_ 315 (7109): 629–634.
Article CAS PubMed PubMed Central Google Scholar * Flores-Lujano J, Perez-Saldivar ML, Fuentes-Panana EM, Gorodezky C, Bernaldez-Rios R, Del Campo-Martinez MA, Martinez-Avalos A,
Medina-Sanson A, Paredes-Aguilera R, De Diego-Flores Chapa J, Bolea-Murga V, Rodriguez-Zepeda MC, Rivera-Luna R, Palomo-Colli MA, Romero-Guzman L, Perez-Vera P, Alvarado-Ibarra M,
Salamanca-Gomez F, Fajardo-Gutierrez A, Mejia-Arangure JM (2009) Breastfeeding and early infection in the aetiology of childhood leukaemia in down syndrome. _Br J Cancer_ 101 (5): 860–864.
Article CAS PubMed PubMed Central Google Scholar * Gart JJ, Nam J (1988) Approximate interval estimation of the ratio of binomial parameters: a review and corrections for skewness.
_Biometrics_ 44 (2): 323–338. Article CAS PubMed Google Scholar * Greaves M (2006) Infection, immune responses and the aetiology of childhood leukaemia. _Nat Rev Cancer_ 6 (3): 193–203.
Article CAS PubMed Google Scholar * Greaves MF (1997) Aetiology of acute leukaemia. _Lancet_ 349 (9048): 344–349. Article CAS PubMed Google Scholar * Greaves MF, Maia AT, Wiemels JL,
Ford AM (2003) leukaemia in twins: lessons in natural history. _Blood_ 102 (7): 2321–2333. Article CAS PubMed Google Scholar * Greenland S (1987) Quantitative methods in the review of
epidemiologic literature. _Epidemiol Rev_ 9: 1–30. Article CAS PubMed Google Scholar * Greenland S, Longnecker MP (1992) Methods for trend estimation from summarised dose–response data,
with applications to meta-analysis. _Am J Epidemiol_ 135 (11): 1301–1309. Article CAS PubMed Google Scholar * Hjalgrim LL, Rostgaard K, Hjalgrim H, Westergaard T, Thomassen H, Forestier
E, Gustafsson G, Kristinsson J, Melbye M, Schmiegelow K (2004) Birth weight and risk for childhood leukaemia in Denmark, Sweden, Norway, and Iceland. _JNCI_ 96 (20): 1549–1556. Article
PubMed Google Scholar * Ibrahem WN, Hasony HJ, Hassan JG (2014) Human parvovirus B19 in childhood acute lymphoblastic leukaemia in Basrah. _JPMA_ 64 (1): 9–12. Google Scholar * Jourdan-Da
Silva N, Perel Y, Mechinaud F, Plouvier E, Gandemer V, Lutz P, Vannier JP, Lamagnere JL, Margueritte G, Boutard P, Robert A, Armari C, Munzer M, Millot F, De Lumley L, Berthou C, Rialland
X, Pautard B, Hemon D, Clavel J (2004) Infectious diseases in the first year of life, perinatal characteristics and childhood acute leukaemia. _Br J Cancer_ 90 (1): 139–145. Article CAS
PubMed PubMed Central Google Scholar * Kerr JR, Barah F, Cunniffe VS, Smith J, Vallely PJ, Will AM, Wynn RF, Stevens RF, Taylor GM, Cleator GM, Eden OB (2003) Association of acute
parvovirus B19 infection with new onset of acute lymphoblastic and myeloblastic leukaemia. _J Clin Pathol_ 56 (11): 873–875. Article CAS PubMed PubMed Central Google Scholar * Kerr JR,
Mattey DL (2015) The role of parvovirus B19 and the immune response in the pathogenesis of acute leukaemia. _Rev Med Virol_ 25 (3): 133–155. Article CAS PubMed Google Scholar * Kinlen L
(1988) Evidence for an infective cause of childhood leukaemia: comparison of a Scottish new town with nuclear reprocessing sites in Britain. _Lancet_ 332 (8624): 1323–1327. Article Google
Scholar * Kinlen L (2006) Childhood leukaemia and ordnance factories in west Cumbria during the Second World War. _Br J Cancer_ 95 (1): 102–106. Article CAS PubMed PubMed Central Google
Scholar * Kinlen L, Doll R (2004) Population mixing and childhood leukaemia: Fallon and other US clusters. _Br J Cancer_ 91 (1): 1–3. Article CAS PubMed PubMed Central Google Scholar
* Kinlen LJ (2012) An examination, with a meta-analysis, of studies of childhood leukaemia in relation to population mixing. _Br J Cancer_ 107 (7): 1163–1168. Article CAS PubMed PubMed
Central Google Scholar * Kwan ML, Buffler PA, Abrams B, Kiley VA (2004) Breastfeeding and the risk of childhood leukaemia: a meta-analysis. _Public Health Rep_ 119 (6): 521–535. Article
PubMed PubMed Central Google Scholar * Lagakos SW (2006) The challenge of subgroup analyses—reporting without distorting. _N Engl J Med_ 354 (16): 1667–1669. Article CAS PubMed Google
Scholar * Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. _Biometrics_ 33 (1): 159–174. Article CAS PubMed Google Scholar * Larsen PS,
Kamper-Jorgensen M, Adamson A, Barros H, Bonde JP, Brescianini S, Brophy S, Casas M, Charles MA, Devereux G, Eggesbo M, Fantini MP, Frey U, Gehring U, Grazuleviciene R, Henriksen TB,
Hertz-Picciotto I, Heude B, Hryhorczuk DO, Inskip H, Jaddoe VW, Lawlor DA, Ludvigsson J, Kelleher C, Kiess W, Koletzko B, Kuehni CE, Kull I, Kyhl HB, Magnus P, Momas I, Murray D, Pekkanen J,
Polanska K, Porta D, Poulsen G, Richiardi L, Roeleveld N, Skovgaard AM, Sram RJ, Strandberg-Larsen K, Thijs C, Van Eijsden M, Wright J, Vrijheid M, Andersen AM (2013) Pregnancy and birth
cohort resources in europe: a large opportunity for aetiological child health research. _Paediatr Perinat Epidemiol_ 27 (4): 393–414. Article PubMed Google Scholar * Lim JYS, Bhatia S,
Robison LL, Yang JJ (2014) Genomics of racial and ethnic disparities in childhood acute lymphoblastic leukaemia. _Cancer_ 120 (7): 955–962. Article PubMed Google Scholar * Lin JN, Lin CL,
Lin MC, Lai CH, Lin HH, Yang CH, Sung FC, Kao CH (2015) Risk of leukaemia in children infected with enterovirus: a nationwide, retrospective, population-based, Taiwanese-registry, cohort
study. _Lancet Oncol_ 16 (13): 1335–1343. Article PubMed Google Scholar * Loutfy SA, Alam El-Din HM, Ibrahim MF, Hafez MM (2006) Seroprevalence of herpes simplex virus types 1 and 2,
Epstein–Barr virus, and cytomegalovirus in children with acute lymphoblastic leukaemia in Egypt. _Saudi Med J_ 27 (8): 1139–1145. PubMed Google Scholar * Ma X, Buffler PA, Wiemels JL,
Selvin S, Metayer C, Loh M, Does MB, Wiencke JK (2005) Ethnic difference in daycare attendance, early infections, and risk of childhood acute lymphoblastic leukaemia. _Cancer Epidemiol
Biomarkers Prev_ 14 (8): 1928–1934. Article PubMed Google Scholar * Ma XM, Urayama K, Chang J, Wiemels JL, Buffler PA (2009) Infection and pediatric acute lymphoblastic leukaemia. _Blood
Cells Mol Dis_ 42 (2): 117–120. Article PubMed Google Scholar * MacArthur AC, McBride ML, Spinelli JJ, Tamaro S, Gallagher RP, Theriault GP (2008) Risk of childhood leukaemia associated
with vaccination, infection, and medication use in childhood: the Cross-Canada Childhood leukaemia Study. _Am J Epidemiol_ 167 (5): 598–606. Article PubMed Google Scholar * Mackenzie J,
Gallagher A, Clayton RA, Perry J, Eden OB, Ford AM, Greaves MF, Jarrett RF (2001) Screening for herpesvirus genomes in common acute lymphoblastic leukaemia. _leukaemia_ 15 (3): 415–421.
Article CAS Google Scholar * Mahjour SB, Ghaffarpasand F, Fattahi MJ, Ghaderi A, Fotouhi Ghiam A, Karimi M (2010) Seroprevalence of human herpes simplex, hepatitis B and Epstein–Barr
viruses in children with acute lymphoblastic leukaemia in southern iran. _Pathol Oncol Res_ 16 (4): 579–582. Article PubMed Google Scholar * Maia Rda R, Wunsch Filho V (2013) Infection
and childhood leukaemia: review of evidence. _Revista de Saude Publica_ 47 (6): 1172–1185. Article PubMed Google Scholar * Martin-Lorenzo A, Hauer J, Vicente-Duenas C, Auer F,
Gonzalez-Herrero I, Garcia-Ramirez I, Ginzel S, Thiele R, Constantinescu SN, Bartenhagen C, Dugas M, Gombert M, Schafer D, Blanco O, Mayado A, Orfao A, Alonso-Lopez D, De Las Rivas J,
Cobaleda C, Garcia-Cenador MB, Garcia-Criado FJ, Sanchez-Garcia I, Borkhardt A (2015) Infection exposure is a causal factor in B-cell precursor acute lymphoblastic leukaemia as a result of
Pax5-inherited susceptibility. _Cancer Discov_ 5 (12): 1328–1343. Article CAS PubMed Google Scholar * Martin RM, Gunnell D, Owen CG, Smith GD (2005) Breast-feeding and childhood cancer:
a systematic review with metaanalysis. _Int J Cancer_ 117 (6): 1020–1031. Article CAS PubMed Google Scholar * McKinney PA, Alexander FE, Nicholson C, Cartwright RA, Carrette J (1991)
Mothers’ reports of childhood vaccinations and infections and their concordance with general practitioner records. _J Public Health Med_ 13 (1): 13–22. Article CAS PubMed Google Scholar
* McKinney PA, Juszczak E, Findlay E, Smith K, Thomson CS (1999) Pre- and perinatal risk factors for childhood leukaemia and other malignancies: a Scottish case control study. _Br J Cancer_
80 (11): 1844–1851. Article CAS PubMed PubMed Central Google Scholar * McNally RJ, Eden TO (2004) An infectious aetiology for childhood acute leukaemia: a review of the evidence. _Br J
Haematol_ 127 (3): 243–263. Article PubMed Google Scholar * Metayer C, Milne E, Clavel J, Infante-Rivard C, Petridou E, Taylor M, Schuz J, Spector LG, Dockerty JD, Magnani C,
Pombo-de-Oliveira MS, Sinnett D, Murphy M, Roman E, Monge P, Ezzat S, Mueller BA, Scheurer ME, Armstrong BK, Birch J, Kaatsch P, Koifman S, Lightfoot T, Bhatti P, Bondy ML, Rudant J,
O'Neill K, Miligi L, Dessypris N, Kang AY, Buffler PA (2013) The Childhood leukaemia International Consortium. _Cancer Epidemiol_ 37 (3): 336–347. Article PubMed PubMed Central
Google Scholar * Neglia JP, Linet MS, Shu XO, Severson RK, Potter JD, Mertens AC, Wen W, Kersey JH, Robison LL (2000) Patterns of infection and day care utilization and risk of childhood
acute lymphoblastic leukaemia. _Br J Cancer_ 82 (1): 234–240. Article CAS PubMed Google Scholar * Nishi M, Miyake H (1989) A case–control study of non-T cell acute lymphoblastic
leukaemia of children in Hokkaido, Japan. _J Epidemiol Community Health_ 43 (4): 352–355. Article CAS PubMed PubMed Central Google Scholar * Paltiel O, Laniado DE, Yanetz R, Deutsch L,
Calderon-Margalit R, Harlap S, Friedlander Y (2006) The risk of cancer following Hospitalisation for infection in infancy: a Population-Based cohort study. _Cancer Epidemiol Biomarkers Prev_
15 (10): 1964–1968. Article PubMed Google Scholar * Perrillat F, Clavel J, Auclerc MF, Baruchel A, Leverger G, Nelken B, Philippe N, Schaison G, Sommelet D, Vilmer E, Hemon D (2002)
Day-care, early common infections and childhood acute leukaemia: a multicentre French case–control study. _Br J Cancer_ 86 (7): 1064–1069. Article CAS PubMed PubMed Central Google
Scholar * Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L (2008) Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. _J Clin
Epidemiol_ 61 (10): 991–996. Article PubMed Google Scholar * Petridou E, Dalamaga M, Mentis A, Skalkidou A, Moustaki M, Karpathios T, Trichopoulos D (2001) Evidence on the infectious
etiology of childhood leukaemia: the role of low herd immunity (Greece). _Cancer Causes Control_ 12 (7): 645–652. Article CAS PubMed Google Scholar * Programme CAS (2014a) _CASP Case
Control Checklist_. CASP: Oxford. * Programme CAS (2014b) _CASP Cohort Study Checklist_. CASP: Oxford. * Riley AW, Duncan GJ (2016) Completing a national birth cohort in the united states.
_JAMA Pediatr_ 170 (9): 829–830. Article PubMed Google Scholar * Roman E, Simpson J, Ansell P, Kinsey S, Mitchell C, McKinney P, Birch J, Greaves M, Eden T (2007) Childhood acute
lymphoblastic leukaemia and infections in the first year of life: a report from the United Kingdom Childhood Cancer Study. _Am J Epidemiol_ 165 (5): 496–504. Article CAS PubMed Google
Scholar * Rosella L, Bowman C, Pach B, Morgan S, Fitzpatrick T, Goel V (2015) The development and validation of a meta-tool for quality appraisal of public health evidence: Meta Quality
Appraisal Tool (MetaQAT). _Public Health_ 136: 57–65. Article Google Scholar * Rosenbaum PF, Buck GM, Brecher ML (2005) Allergy and infectious disease histories and the risk of childhood
acute lymphoblastic leukaemia. _Paediatr Perinat Epidemiol_ 19 (2): 152–164. Article PubMed Google Scholar * Rudant J, Lightfoot T, Urayama KY, Petridou E, Dockerty JD, Magnani C, Milne
E, Spector LG, Ashton LJ, Dessypris N, Kang AY, Miller M, Rondelli R, Simpson J, Stiakaki E, Orsi L, Roman E, Metayer C, Infante-Rivard C, Clavel J (2015) Childhood acute lymphoblastic
leukaemia and indicators of early immune stimulation: a Childhood leukaemia International Consortium study. _Am J Epidemiol_ 181 (8): 549–562. Article PubMed PubMed Central Google Scholar
* Rudant J, Orsi L, Menegaux F, Petit A, Baruchel A, Bertrand Y, Lambilliotte A, Robert A, Michel G, Margueritte G, Tandonnet J, Mechinaud F, Bordigoni P, Hemon D, Clavel J (2010)
Childhood acute leukaemia, early common infections, and allergy: The ESCALE Study. _Am J Epidemiol_ 172 (9): 1015–1027. Article PubMed Google Scholar * Salonen MJH, Siimes MA, Salonen EM,
Vaheri A, Koskiniemi M (2002) Antibody status to HHV-6 in children with leukaemia. _leukaemia_ 16 (4): 716–719. Article CAS Google Scholar * Schlehofer B, Blettner M, Geletneky K, Haaf
HG, Kaatsch P, Michaelis J, MuellerLantzsch N, Niehoff D, Winkelspecht B, Wahrendorf J, Schlehofer JR (1996) Sero-epidemiological analysis of the risk of virus infections for childhood
leukaemia. _Int J Cancer_ 65 (5): 584–590. Article CAS PubMed Google Scholar * Schuz J, Kaletsch U, Meinert R, Kaatsch P, Michaelis J (1999) Association of childhood leukaemia with
factors related to the immune system. _Br J Cancer_ 80 (3-4): 585–590. Article CAS PubMed PubMed Central Google Scholar * Simpson J, Smith A, Ansell P, Roman E (2007) Childhood
leukaemia and infectious exposure: a report from the United Kingdom Childhood Cancer Study (UKCCS). _Eur J Cancer_ 43 (16): 2396–2403. Article PubMed Google Scholar * Stroup DF, Berlin
JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting.
Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. _JAMA_ 283 (15): 2008–2012. Article CAS PubMed Google Scholar * Surico G, Muggeo P (2005) Epstein–Barr virus
infection at the onset of acute lymphoblastic leukaemia in children. _Int Cancer J Austral Asia_ 4 (1): 19–24. Google Scholar * Swaminathan S, Klemm L, Park E, Papaemmanuil E, Ford A, Kweon
SM, Trageser D, Hasselfeld B, Henke N, Mooster J, Geng H, Schwarz K, Kogan SC, Casellas R, Schatz DG, Lieber MR, Greaves MF, Muschen M (2015) Mechanisms of clonal evolution in childhood
acute lymphoblastic leukaemia. _Nat Immunol_ 16 (7): 766–774. Article CAS PubMed PubMed Central Google Scholar * Tesse R, Santoro N, Giordano P, Cardinale F, De Mattia D, Armenio L
(2009) Association between DEFB1 gene haplotype and herpes viruses seroprevalence in children with acute lymphoblastic leukaemia. _Pediatr Hematol Oncol_ 26 (8): 573–582. Article CAS
PubMed Google Scholar * Till M, Rapson N, Smith PG (1979) Family studies in acute leukaemia in childhood: a possible association with autoimmune disease. _Br J Cancer_ 40 (1): 62–71.
Article CAS PubMed PubMed Central Google Scholar * Urayama KY, Buffler PA, Gallagher ER, Ayoob JM, Ma X (2010) A meta-analysis of the association between day-care attendance and
childhood acute lymphoblastic leukaemia. _Int J Epidemiol_ 39 (3): 718–732. Article PubMed PubMed Central Google Scholar * van Steensel-Moll HA, Valkenburg HA, van Zanen GE (1986)
Childhood leukaemia and infectious diseases in the first year of life: a Register-Based Case–Control Study. _Am J Epidemiol_ 124 (4): 590–594. Article CAS PubMed Google Scholar *
Vestergaard TR, Rostgaard K, Grau K, Schmiegelow K, Hjalgrim H (2013) Hospitalisation for infection prior to diagnosis of acute lymphoblastic leukaemia in children. _Pediatric Blood Cancer_
60 (3): 428–432. Article PubMed Google Scholar * Viechtbauer W (2010) Conducting meta-analyses in R with the metafor Package. _J Stat Softw_ 36 (3): 48. Article Google Scholar *
Whitehead TP, Metayer C, Wiemels JL, Singer AW, Miller MD (2016) Childhood leukaemia and primary prevention. _Curr Prob Pediatric Adolesc Health care_ 46 (10): 317–352. Article Google
Scholar * Wiemels J (2012) Perspectives on the causes of childhood leukaemia. _Chemico-Biol Interact_ 196 (3): 59–67. Article CAS Google Scholar * Young NS, Brown KE (2004) Parvovirus
B19. _N Engl J Med_ 350 (6): 586–597. Article CAS PubMed Google Scholar * Zaki ME, Hassan SA, Seleim T, Lateef RA (2006) Parvovirus B19 infection in children with a variety of
hematological disorders. _Hematology_ 11 (4): 261–266. Article CAS Google Scholar * Zaki MES, Ashray RE (2010) Clinical and hematological study for Parvovirus b19 infection in children
with acute leukaemia. _Int J Lab Hematol_ 32 (2): 159–166. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank Thomasin Adams-Webber for her
assistance with the citation searches of the electronic databases. Ms Adams-Webber is a trained librarian with expertise in conducting searches for systematic reviews in clinical research.
The study forms part of Jeremiah Hwee’s doctoral dissertation. This work was supported by the Canadian Institutes of Health Research Frederick Banting and Charles Best Canada Graduate
Scholarship to Honour Nelson Mandela—Doctoral award (JH). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Dalla Lana School of Public Health, University of Toronto, Health Sciences Building,
155 College Street, Toronto, ON, Canada Jeremiah Hwee, Christopher Tait, Jeffrey C Kwong, Rinku Sutradhar & Jason D Pole * Division of Haematology/Oncology, Department of Pediatrics, The
Hospital for Sick Children, 555 University Avenue, Toronto, ON, Canada Lillian Sung * Program in Child Health Evaluative Sciences, The Hospital for Sick Children, Peter Gilgan Centre for
Research and Learning, 686 Bay Street, Toronto, ON, Canada Lillian Sung * Institute of Health Policy, Management and Evaluation, University of Toronto, Health Sciences Building, 155 College
Street, Toronto, ON, Canada Lillian Sung * Institute for Clinical Evaluative Sciences, 2075 Bayview Avenue, Toronto, ON, Canada Jeffrey C Kwong & Rinku Sutradhar * Department of Family
and Community Medicine, University of Toronto, Toronto, ON, Canada Jeffrey C Kwong * Public Health Ontario, Toronto, ON, Canada Jeffrey C Kwong * Toronto Western Family Health Team,
University Health Network, Toronto, ON, Canada Jeffrey C Kwong * Pediatric Oncology Group of Ontario, 480 University Avenue, Suite 1014, Toronto, ON, Canada Jason D Pole Authors * Jeremiah
Hwee View author publications You can also search for this author inPubMed Google Scholar * Christopher Tait View author publications You can also search for this author inPubMed Google
Scholar * Lillian Sung View author publications You can also search for this author inPubMed Google Scholar * Jeffrey C Kwong View author publications You can also search for this author
inPubMed Google Scholar * Rinku Sutradhar View author publications You can also search for this author inPubMed Google Scholar * Jason D Pole View author publications You can also search for
this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Jeremiah Hwee. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL
INFORMATION This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative
Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. Supplementary Information accompanies this paper on British Journal of Cancer website SUPPLEMENTARY INFORMATION
SUPPLEMENTARY FIGURE LEGENDS (DOCX 12 KB) SUPPLEMENTARY TABLE 1 (DOCX 16 KB) SUPPLEMENTARY TABLE 2 (DOCX 23 KB) SUPPLEMENTARY TABLE 3 (DOCX 24 KB) SUPPLEMENTARY TABLE 4 (DOCX 17 KB)
SUPPLEMENTARY FIGURE 1 (JPG 53 KB) SUPPLEMENTARY FIGURE 2 (JPG 128 KB) SUPPLEMENTARY FIGURE 3 (JPG 213 KB) RIGHTS AND PERMISSIONS From twelve months after its original publication, this work
is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hwee, J., Tait, C., Sung, L. _et al._ A systematic review and meta-analysis of the association between childhood infections and
the risk of childhood acute lymphoblastic leukaemia. _Br J Cancer_ 118, 127–137 (2018). https://doi.org/10.1038/bjc.2017.360 Download citation * Received: 23 June 2017 * Revised: 19
September 2017 * Accepted: 21 September 2017 * Published: 24 October 2017 * Issue Date: January 2018 * DOI: https://doi.org/10.1038/bjc.2017.360 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * childhood acute lymphoblastic leukaemia * infection * aetiology * systematic review and meta-analysis
