Survival of patients who develop solid tumors following hematopoietic stem cell transplantation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Allogeneic hematopoietic cell transplantation is associated with late adverse effects of therapy, including secondary solid cancers. Most reports address risk factors; however,
outcomes after secondary solid cancer development are incompletely described. Our objective was to estimate survival probabilities for transplant recipients dependent on secondary solid
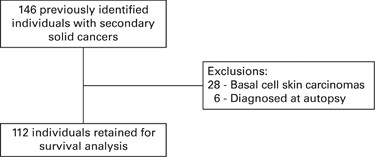
cancer subtype. We used a previously identified and published cohort who developed secondary solid cancers following allogeneic transplant. Follow-up for these 112 previously identified
patients was extended and their survival probabilities were studied. Median duration of follow-up from the development of secondary cancer for survivors was 11.9 years (range: 0.8–23.4) and
75% were followed >7.0 years. The 5- and 10-year overall survival probabilities were 50% (95% confidence interval (CI): 41–60) and 46% (95% CI: 37–57), respectively. Overall survival
varied by secondary cancer type. Secondary cancer was the cause of death in most patients who died following development of melanoma, central nervous system, oral cavity, thyroid, lung,
lower gastrointestinal tract and bone cancers. Extended follow-up allowed for the most comprehensive longitudinal evaluation to date of this rare condition. These findings will enhance
clinicians' ability to predict outcomes and counsel transplant survivors who develop secondary solid cancers. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only
$21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS EFFECT OF AUTOLOGOUS
HEMATOPOIETIC STEM CELL TRANSPLANT ON THE DEVELOPMENT OF SECOND PRIMARY MALIGNANCIES IN MULTIPLE MYELOMA PATIENTS Article Open access 07 January 2021 LONG-TERM OUTCOMES OF ALLOGENEIC STEM
CELL TRANSPLANT IN MULTIPLE MYELOMA Article Open access 18 August 2023 HOW RISKY IS A SECOND ALLOGENEIC STEM CELL TRANSPLANTATION? Article Open access 25 June 2024 REFERENCES * Gratwohl A,
Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A _et al_. Hematopoietic stem cell transplantation: a global perspective. _JAMA_ 2010; 303: 1617–1624. Article CAS Google Scholar *
Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG _et al_. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report
from the Bone Marrow Transplant Survivor Study. _Blood_ 2007; 110: 3784–3792. Article CAS Google Scholar * Socie G, Stone JV, Wingard JR, Weisdorf D, Henslee-Downey PJ, Bredeson C _et
al_. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. _N Engl J Med_
1999; 341: 14–21. Article CAS Google Scholar * Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D _et al_. Long-term survival and late deaths after allogeneic
hematopoietic cell transplantation. _J Clin Oncol_ 2011; 29: 2230–2239. Article Google Scholar * Geenen MM, Cardous-Ubbink MC, Kremer LC, Van den Bos C, Van der Pal HJ, Heinen RC _et al_.
Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. _JAMA_ 2007; 297: 2705–2715. Article CAS Google Scholar * Sun CL, Francisco L, Kawashima T,
Leisenring W, Robison LL, Baker KS _et al_. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor
Study. _Blood_ 2010; 116: 3129–3139; quiz 377. Article CAS Google Scholar * Bresters D, van Gils IC, Kollen WJ, Ball LM, Oostdijk W, van der Bom JG _et al_. High burden of late effects
after haematopoietic stem cell transplantation in childhood: a single-centre study. _Bone Marrow Transplant_ 2010; 45: 79–85. Article CAS Google Scholar * Oeffinger KC, Mertens AC, Sklar
CA, Kawashima T, Hudson MM, Meadows AT _et al_. Chronic health conditions in adult survivors of childhood cancer. _N Engl J Med_ 2006; 355: 1572–1582. Article CAS Google Scholar * Hudson
MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR _et al_. Clinical ascertainment of health outcomes among adults treated for childhood cancer. _JAMA_ 2013; 309: 2371–2381.
Article CAS Google Scholar * Majhail NS . Old and new cancers after hematopoietic-cell transplantation. _Hematology Am Soc Hematol Educ Program_ 2008; 2008: 142–149. Article Google
Scholar * Rizzo JD, Curtis RE, Socie G, Sobocinski KA, Gilbert E, Landgren O _et al_. Solid cancers after allogeneic hematopoietic cell transplantation. _Blood_ 2009; 113: 1175–1183.
Article CAS Google Scholar * Hudson MM . A model for care across the cancer continuum. _Cancer_ 2005; 104: 2638–2642. Article Google Scholar * Gallagher G, Forrest DL . Second solid
cancers after allogeneic hematopoietic stem cell transplantation. _Cancer_ 2007; 109: 84–92. Article Google Scholar * Shimada K, Yokozawa T, Atsuta Y, Kohno A, Maruyama F, Yano K _et al_.
Solid tumors after hematopoietic stem cell transplantation in Japan: incidence, risk factors and prognosis. _Bone Marrow Transplant_ 2005; 36: 115–121. Article CAS Google Scholar * Bhatia
S, Louie AD, Bhatia R, O'Donnell MR, Fung H, Kashyap A _et al_. Solid cancers after bone marrow transplantation. _J Clin Oncol_ 2001; 19: 464–471. Article CAS Google Scholar * Kolb
HJ, Socie G, Duell T, Van Lint MT, Tichelli A, Apperley JF _et al_. Malignant neoplasms in long-term survivors of bone marrow transplantation. Late Effects Working Party of the European
Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. _Ann Intern Med_ 1999; 131: 738–744. Article CAS Google Scholar * Baker KS, DeFor TE,
Burns LJ, Ramsay NK, Neglia JP, Robison LL . New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. _J Clin Oncol_ 2003; 21:
1352–1358. Article Google Scholar * Ringden O, Brazauskas R, Wang Z, Ahmed I, Atsuta Y, Buchbinder D _et al_. Second solid cancers after allogeneic hematopoietic cell transplantation using
reduced-intensity conditioning. _Biol Blood Marrow Transplant_ 2014; 20: 1777–1784. Article Google Scholar * Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB _et al_. Solid
cancers after bone marrow transplantation. _N Engl J Med_ 1997; 336: 897–904. Article CAS Google Scholar * Klein JP, Moeschberger ML . _Survival Analysis: Techniques of Censored and
Truncated Data_. 2nd ed. Springer-Verlag: New York, NY, USA, 2003. Google Scholar * Rizzo JD, Wingard JR, Tichelli A, Lee SJ, Van Lint MT, Burns LJ _et al_. Recommended screening and
preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, Center for
International Blood and Marrow Transplant Research, and the American Society for Blood and Marrow Transplantation (EBMT/CIBMTR/ASBMT). _Bone Marrow Transplant_ 2006; 37: 249–261. Article
CAS Google Scholar * Howlader N, Noon AM, Krapcho M, Garshell J, Miller D, Altekruse SF _et al_ (eds) _SEER Cancer Statistics Review, 1975–2011_. National Cancer Institute: Bethesda, MD,
http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014. Download references ACKNOWLEDGEMENTS We thank Drs M Kelly, R
Tower, J Panepinto, A Brandow, M Eapen, D Margolis, A Maguire and D Mulrooney for their support of and insight into this project. The CIBMTR is supported by Public Health Service
Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious
Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants
N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Cancer Survivorship, Department of Oncology, St Jude
Children’s Research Hospital, Memphis, TN, USA M J Ehrhardt * Division of Biostatistics, Institute for Health and Society, Medical College of Wisconsin, Milwaukee, WI, USA R Brazauskas *
Department of Medicine, CIBMTR (Center for International Blood and Marrow Transplant Research), Froedtert and the Medical College of Wisconsin Clinical Cancer Center, Milwaukee, WI, USA R
Brazauskas, W He, J D Rizzo & B E Shaw Authors * M J Ehrhardt View author publications You can also search for this author inPubMed Google Scholar * R Brazauskas View author publications
You can also search for this author inPubMed Google Scholar * W He View author publications You can also search for this author inPubMed Google Scholar * J D Rizzo View author publications
You can also search for this author inPubMed Google Scholar * B E Shaw View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR
Correspondence to B E Shaw. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Ehrhardt, M., Brazauskas, R., He, W. _et al._ Survival of patients who develop solid tumors following hematopoietic stem cell transplantation. _Bone Marrow Transplant_ 51, 83–88
(2016). https://doi.org/10.1038/bmt.2015.203 Download citation * Received: 09 January 2015 * Revised: 04 June 2015 * Accepted: 17 June 2015 * Published: 14 September 2015 * Issue Date:
January 2016 * DOI: https://doi.org/10.1038/bmt.2015.203 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
