Hemojuvelin regulates the innate immune response to peritoneal bacterial infection in mice
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Hereditary hemochromatosis and iron imbalance are associated with susceptibility to bacterial infection; however, the underlying mechanisms are poorly understood. Here, we performed in vivo
bacterial infection screening using several mouse models of hemochromatosis, including Hfe (Hfe−/−), hemojuvelin (Hjv−/−), and macrophage-specific ferroportin-1 (Fpn1fl/fl;LysM-Cre+)
knockout mice. We found that Hjv−/− mice, but not Hfe−/− or Fpn1fl/fl;LysM-Cre+ mice, are highly susceptible to peritoneal infection by both Gram-negative and Gram-positive bacteria.
Interestingly, phagocytic cells in the peritoneum of Hjv−/− mice have reduced bacterial clearance, IFN-γ secretion, and nitric oxide production; in contrast, both cell migration and
phagocytosis are normal. Expressing Hjv in RAW264.7 cells increased the level of phosphorylated Stat1 and nitric oxide production. Moreover, macrophage-specific Hjv knockout mice are
susceptible to bacterial infection. Finally, we found that Hjv facilitates the secretion of IFN-γ via the IL-12/Jak2/Stat4 signaling pathway. Together, these findings reveal a novel
protective role of Hjv in the early stages of antimicrobial defense.
In the 1960s, researchers first suggested a link between iron metabolism and the immune system [1]. Since then, many iron-related genes have been found to play a role in immune function. For
example, the proteins lactoferrin, hepcidin, and Hfe have all been found to modulate the host defense against bacterial infection [2–6]. Moreover, the expression of several genes involved
in iron metabolism, including hemojuvelin (HJV) [7], hepcidin (HAMP) [8], and ferroportin1 (FPN1) [9, 10], are modulated during inflammation. Interestingly, a recent case report described a
patient with hemochromatosis who died from bacterial infection [11]. These findings prompted us to investigate the putative role of hemochromatosis-related genes in bacterial infection. We
therefore screened several hemochromatosis mouse models for their immune phenotype and susceptibility to bacterial infection.
HJV is a bone morphogenetic protein (BMP) co-receptor that regulates the expression of hepcidin [12]. In humans, mutations in the HJV gene cause juvenile hemochromatosis [13]. In mice,
deleting Hjv expression causes a similar iron-overload phenotype [7, 14]. HJV is also a member of the repulsive guidance molecule (RGM) family [15]. However, the role of HJV in the immune
response is currently unknown. Recent studies suggest that two other RGM family members—RGMa and RGMb—play a role in immune regulation. For example, RGMa is found in bone marrow-derived
dendritic cells, where it modulates T cell responses [16]. RGMb is strongly expressed in macrophages, and increased IL-6 levels were measured in macrophages isolated from Rgmb-knockout mice
[17]. Notably, the Hjv gene (also known as RGMc) is also expressed in macrophages [17], and studies suggest that lipopolysaccharide (LPS) stimulation induces organ-specific patterns of Hjv
expression [7]. However, whether Hjv plays a role in the immune system’s response to bacterial infection is currently unknown. Here, we used an acute infection mouse model to investigate the
potential immunomodulatory roles of hemochromatosis genes. Our results indicate that Hjv plays a major role in mediating the host innate immune response to bacterial infection.
To investigate the function of hemochromatosis-related genes in response to acute bacterial infection, we administered an intraperitoneal (i.p.) injection of a lethal dose of the
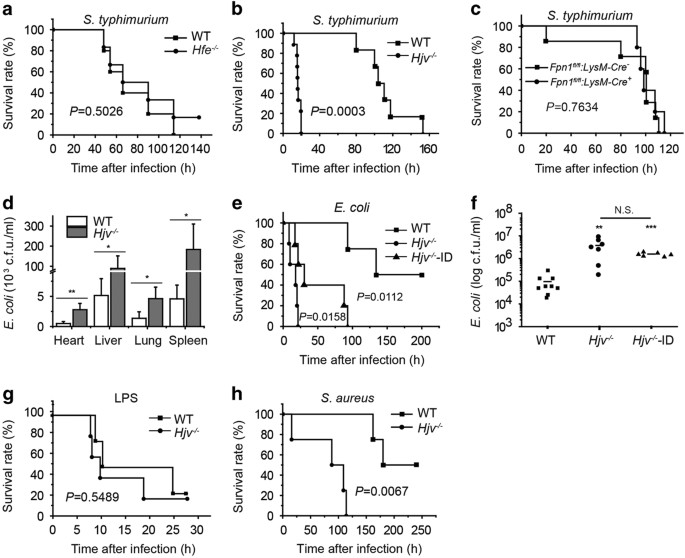
Gram-negative bacterium Salmonella typhimurium (S. typhimurium) [5, 18 ] to the following hemochromatosis mouse models: Hfe-knockout (Hfe−/−) mice (Figure 1a), hemojuvelin-knockout (Hjv−/−)
mice (Figure 1b), and macrophage-specific ferroportin1-knockout (Fpn1fl/fl;LysM-Cre+) mice (Figure 1c). Although all three models are reported to develop systemic iron overload [9], only the
Hjv−/− mice were highly susceptible to S. typhimurium infection, reaching 100% mortality within 20 h of infection.
Hjv confers protection against Gram-positive and Gram-negative bacterial infections. (a–c) Hfe−/− (a), Hjv−/− (b), and Fpn1fl/fl;LysM-Cre+ mice (c)—or their respective controls—were given an
i.p. injection of S. typhimurium (106 CFU), and survival was plotted using a Kaplan-Meier curve. N=5–8 mice/group. (d) Bacterial CFU values were measured in the heart, liver, spleen, and
lung tissues of Hjv−/− and wild-type mice 12 h after an i.p. injection of Escherichia coli. *P
