High frequency of copy number imbalances in rubinstein–taybi patients negative to crebbp mutational analysis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Rubinstein–Taybi syndrome (RSTS) is a rare autosomal dominant disorder characterised by facial dysmorphisms, growth and psychomotor development delay, and skeletal defects. The
known genetic causes are point mutations or deletions of the _CREBBP_ (50–60%) and _EP300_ (5%) genes. To detect chromosomal rearrangements indicating novel positional candidate RSTS genes,
we used a-CGH to study 26 patients fulfilling the diagnostic criteria for RSTS who were negative at fluorescence _in situ_ hybridisation analyses of the _CREBBP_ and _EP300_ regions, and
direct sequencing analyses of the _CREBBP_ gene. We found seven imbalances (27%): four _de novo_ and three inherited rearrangements not reported among the copy number variants. A _de novo_
7p21.1 deletion of 500 kb included the _TWIST1_ gene, a suggested candidate for RSTS that is responsible for the Saethre–Chotzen syndrome, an entity that enters in differential diagnosis
with RSTS. A similar issue of differential diagnosis was raised by a large 4.3 Mb 2q22.3q23.1 deletion encompassing _ZEB2_, the gene responsible for the Mowat–Wilson syndrome, whose signs
may overlap with RSTS. Positional candidate genes could not be sought in the remaining pathogenetic imbalances, because of the size of the involved region (a 9 Mb 2q24.3q31.1 deletion)
and/or the relative paucity of suitable genes (a 5 Mb 3p13p12.3 duplication). One of the inherited rearrangements, the 17q11.2 379Kb duplication, represents the reciprocal event of the
deletion underlying an overgrowth syndrome, both being mediated by the NF1-REP-P1 and REP-P2 sub-duplicons. The contribution of this and the other detected CNVs to the clinical RSTS
phenotype is difficult to assess. SIMILAR CONTENT BEING VIEWED BY OTHERS GENOMIC BALANCING ACT: DECIPHERING DNA REARRANGEMENTS IN THE COMPLEX CHROMOSOMAL ABERRATION INVOLVING 5P15.2, 2Q31.1,
AND 18Q21.32 Article Open access 10 September 2024 DE NOVO 2Q36.3Q37.1 DELETION ENCOMPASSING _TRIP12_ AND _NPPC_ YIELDS DISTINCT PHENOTYPES Article Open access 01 June 2020 16P13.11P11.2
TRIPLICATION SYNDROME: A NEW RECOGNIZABLE GENOMIC DISORDER CHARACTERIZED BY OPTICAL GENOME MAPPING AND WHOLE GENOME SEQUENCING Article 07 April 2022 INTRODUCTION Rubinstein–Taybi syndrome
(RSTS, MIM #180849) is an autosomal dominant disease that occurs in 1 out of 125 000 births. Affected patients are characterised by growth and psychomotor development delay because of the
major involvement of the skeletal and central nervous systems. The main skeletal features are radially diverted phalanges (in one-third of the cases) and broad and duplicated distal
phalanges of the thumbs and halluces (99% of the patients), which are hallmark signs of the syndrome. Typical facial dysmorphisms such as down-slanting palpebral fissures and a prominent
beaked nose help clinical geneticists make a diagnosis.1 The patients are also at increased risk of tumours,2 especially those involving the epidermis such as pilomatrixomas3 and epidermal
nevi.4 RSTS is caused by mutations in two genes: cAMP response element-binding protein (_CREBBP_, also known as ‘_CBP_’) localised at 16p13.3,5 and E1A-associated protein p300 (_EP300_),
localised at 22q13.6 Both genes encode histone acetyltransferases (HATs), transcriptional co-activators that are involved in cell processes such as growth, differentiation, DNA repair,
apoptosis and many others,7 and which also have an important role in the development of the skeletal and nervous central systems, thus accounting for the growth and psychomotor development
delay typical of RSTS patients. Various techniques have been used to identify the genetic lesion underlying RSTS, including fluorescence _in situ_ hybridisation (FISH),8, 9, 10, 11 real-time
quantitative PCR,12 multiplex ligation-dependent probe amplification (MLPA),13 denaturing high performance liquid chromatography (DHPLC)14 and sequencing.15, 16 Deletions of part or all of
the _CREBBP_ gene and flanking regions (also in mosaic condition),11 may account for 5–10% of the cases, whereas frameshift, nonsense, splice-site and missense point mutations (in decreasing
order of prevalence) are found in approximately 46–51%;17 there have also been reports of a few gene-disrupting translocations and inversions.10 _EP300_ mutations are comparatively rare as
only six have been described so far, in six patients with a mild clinical presentation.6, 18, 19, 20 The pathological mechanism has been clarified in approximately 60% of the cases, but
multiple cryptic mutational mechanisms of the major _CBP_ gene may be overlooked, including mosaic point mutations, mutations affecting the 5′ and 3′ UTRs, promoter epimutations and
alterations at post- transcriptional or translational levels. In brief, RSTS is a heterogeneous disorder because of at least two different genes, thus allowing speculation that other genes
may also be involved and lead to the observed spectrum of clinical presentations. Array comparative genomic hybridisation (a-CGH) is a powerful means of interrogating the overall genome for
copy number alterations, but has so far only been used to analyse the _CREBBP_ region in RSTS patients.21 The aim of this genome-wide assay was to identify rearrangements that might underlie
RSTS-like phenotypes, and possibly detect positional candidate genes, in 26 Italian patients with a clinical diagnosis of RSTS who were negative at FISH analyses of the _CREBBP_ and _EP300_
regions and sequencing analyses of the _CREBBP_ gene. We found seven imbalances (four _de novo_ and three inherited rearrangements) and discuss their implications in RSTS and in related
syndromic entities. MATERIALS AND METHODS The 26 patients analysed by means of array CGH had a clinical diagnosis of RSTS, and were negative at FISH analyses of the _CREBBP_ and _EP300_
regions, and direct sequencing analyses of the _CREBBP_ gene. Genomic DNA was isolated from the patients’ peripheral blood samples using a QIAamp DNA Blood Kit (Qiagen, Hilden, Germany).
ARRAY CGH ANALYSIS All of the patients were analysed using the 4 × 44K genome-wide chip (Agilent Technologies, Santa Clara, CA, USA), which has an average resolution of 100 kb. The parents
of the carriers of genomic imbalances were also analysed. Aliquots of 500 ng of patient and reference DNAs (Promega Corporation, Madison, WI, USA) were double-digested with _Rsa_I and _Alu_I
(Promega) for 2 h at 37 °C. After heat inactivation of restriction endonucleases at 65 °C for 20 min, each digested sample was labelled by means of the Genomic DNA Labelling Kit PLUS
(Agilent Technologies) for 2 h at 37 °C, using Cy5-dUTP for the patient DNAs and Cy3-dUTP for the reference DNAs. After purification from non-incorporated fluorochromes, the DNAs were
properly combined and denatured for 3 min at 95 °C. After 30 min pre-annealing with 5 _μ_g of Cot-1 DNA, the samples were hybridised at 65 °C for 24 h with rotation, and then the
non-hybridised DNA was removed by means of two post-hybridisation washings. Images of the chips were acquired using the Agilent scanner and analysed by means of Feature Extraction 9.5
software 9.5 (Agilent); the results were graphically represented using CGH Analytics 3.5 software (Agilent). FLUORESCENCE _IN SITU_ HYBRIDISATION The chromosome preparations were obtained
using standard cytogenetic techniques. Briefly, phytohemagglutinin(PHA)-stimulated peripheral blood lymphocytes were set up in culture using the ‘Synchro’ Chromosome Kit (Celbio, Milan,
Italy) and modified RPMI (Irvine Scientific, Santa Ana, CA, USA) plus 5% fetal calf serum (Gibco LTD, Paisley, Scotland). The cultures were stopped with colchicine after 72 h. The CREBBP and
EP300 encompassing BAC probes were selected on the basis of their physical location (http://www.genome.ucsc.edu/): details are reported in Gervasini _et al_, 2007. DNA was isolated from
liquid cultures using a plasmid purification kit (Nucleobond PC20, Macherey-Nagel, GmbH & Co.KG, Duren, Germany). The BAC clones were labelled with digoxigenin-11-dUTP or biotin-16-dUTP
(Roche Diagnostics, Mannheim, Germany) using a nick translation kit (Roche Diagnostics). The FISH experiments were performed using standard procedures.22 The chromosomes were counterstained
with DAPI in antifade Vectashield Mounting Medium (Vector Laboratories Inc., Burlingame, CA, USA), and visualised using a Leitz DM-RB microscope (Leica Microsystems GmbH, Wetzlar, Germany)
equipped for DAPI and FITC/TRITC epifluorescence optics. The images were captured by means of a CCD camera High Performance CCD Camera (COHU, Poway, CA, USA), and visualised using McProbe
software (Applied Imaging, PowerGene, League City, TX, USA). MICROSATELLITE SEGREGATION ANALYSIS Segregation from parents to probands was analysed using the D2S2188, D2S399, D3S3653 and
D3S4533 fluorescent dye-labelled microsatellite markers, with the fluorescence being detected by means of an ABI 3100 sequencer. ABI PRISM software Genescan (Applied Biosystem, Foster City,
CA, USA) was used for gel analysis. SNP SEQUENCE ANALYSIS The DNA direct sequencing of the SNPs rs2084674, rs 34729008 and rs2084674 was performed by means of an ABI PRISM 3130 sequencer
(Applied Biosystem), and the electropherograms were analysed using Chromas Pro version 1.42 software (Technelysium Pty LTD, Australia). RESULTS OVERVIEW OF ARRAY CGH FINDINGS The 26 RSTS
patients (14 men and 12 women aged between 6 months and 42 years) had clinical diagnoses ranging from definite (consistent) to probable (suggestive and within the spectrum) or possible
(borderline or RSTS-like). This cohort was selected because the patients were negative for CREBBP point mutations and chromosomal rearrangements affecting the CREBBP and EP300 regions, and
thus consistent with our main aims of detecting genomic regions with sequence gains or losses (to be analysed in further detail in order to refine the clinical diagnosis) and identifying
novel candidate RSTS genes. Seven of the 26 patients carried DNA copy number alterations, including four deletions and three duplications: the _de novo_ imbalances were two large deletions
of chromosome 2q (one encompassing 9 Mb at 2q24.3q31.1 and the other 4.3 Mb at 2q22.3q23.1), a small deletion of chromosome 7p21.1, and a 5 Mb duplication at 3p13p12.3 (Table 1). Table 2
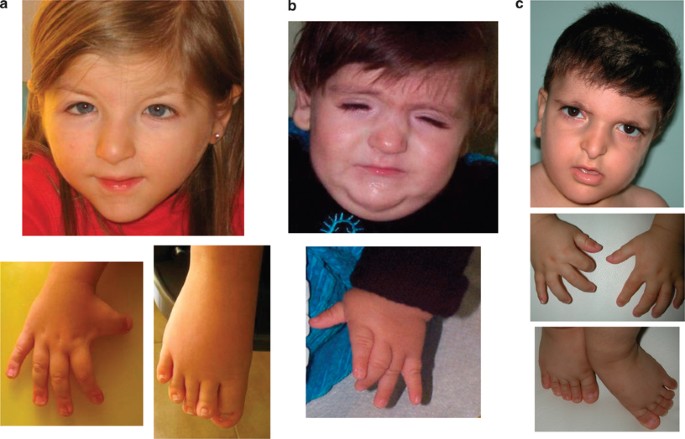
shows the clinical signs of these four cases, and Figure 1 shows the facial appearance and typical right thumb/hallux of three of them. DETAILS REGARDING THE GENOMIC REGIONS INVOLVED IN THE
_DE NOVO_/INHERITED IMBALANCES PATIENT 51 The 4.3 Mb 2q22.3q23.1 deletion was confirmed by FISH using the CTD2162B21 BAC clone. Segregation analysis of SNPs rs2084674 (and rs34729008 mapping
within the deletion) showed that the deletion arose _de novo_ on the paternally contributed chromosome as independently confirmed by array CGH of the parents’ DNA (data not shown). Six
_RefSeq_ genes are located in the deleted interval, including the zinc finger homeobox 1B _ZEB2_ that is responsible for Mowat–Wilson syndrome (MWS) (OMIM#235730) (Figure 2a). PATIENT 14 The
9 Mb 2q24.3q31.1 deletion involves >50 _RefSeq_ genes and the _HOXD_ gene cluster in its distal _HOXD13_ element (Figure 2b). The distal breakpoint was validated and characterised by
means of double-colour metaphase FISH using the CTD-2226C5 (specific for the whole _HOXD_ cluster) and RP11-892L20 BAC (covering the upstream regulatory region), which respectively map
outside and inside the deletion. The same BAC array-CGH and FISH analyses were applied to both parents and confirmed the _de novo_ origin of the deletion. Segregation analysis using
microsatellites D2S2188 and D2S399 (both mapping within the deletion) showed that the deletion arose _de novo_ on the paternally contributed chromosome (data not shown). Using the RP11-471A5
and RP11892L20 BACs (mapping inside the deletion) and CTD-2226B10 (mapping outside) to study the metaphases/nuclei of the father's chromosomes allowed us to exclude the presence of an
inversion predisposing the region to break (data not shown). Previous karyotyping also revealed the presence in the patient of a marker chromosome (data not shown) that was not characterised
in detail. The genes localised within the deleted region that are worth noting include: (i) _HOXD13_, which encodes a transcription factor that has an important role in morphogenesis; (ii)
the _DLX1_ and _DLX2_ genes encoding proteins that may have a role in controlling craniofacial patterning, as well as in the differentiation and survival of inhibitory neurons in the
forebrain; and (iii) the gene for activating transcription factor 2 (_ATF2_, also called _CREBP1_ or _CREB2_) whose product stimulates the CRE (cAMP-responsive element)-dependent genes and
has HAT activity specific for histones H2B and H4. PATIENT 61 The 3p13p12.3 duplication spans 5.1 Mb and harbours seven _RefSeq_ genes (Figure 2c). Segregation analysis using microsatellites
D3S3653 and D3S4533 (mapping within the large duplicated segment) showed its _de novo_ origin on the maternally contributed chromosome, in line with the ‘normal’ array CGH profile of both
parents’ DNA. The duplicated genes of possible pathogenetic relevance are the roundabout, axon guidance receptor, homologue 2 (_ROBO2_) gene, which encodes a protein receptor for SLIT
Drosophila homologue 2 (_SLIT2_) that is known to function in axon guidance and cell migration, and the contactin 3 (_CNTN3_), a member of the contactin family that mediates cell surface
interactions during nervous system development. PATIENT 58 The small deletion of 466 kb in 7p21.1 (Figure 2d) affects the region containing _TWIST1_, the causative gene of Saethre–Chotzen
syndrome (SCS) (OMIM#101400) and interrupts _HDAC9_, a gene coding for a protein whose function is the opposite of that of _CREBBP_. The deletion was validated by FISH analysis using BAC
clone CTD-2050C8, and its _de novo_ origin was confirmed by parent's analysis. PATIENTS 45, 78 AND 29 The deletion of patient 78 (which spans 1.2 Mb in 18q22.1) and the 5.5 Mb 2q34q35
duplication of patient 45, shown by array CGH to be also carried by the patients’ fathers, involve gene-poor regions. A bioinformatic search (Human Genome Segmental Duplication Database
http://projects.tcag.ca/humandup/) revealed that these regions are not numbered copy number polymorphisms. The duplication carried by patient 29 (Figure 3) only affects 379 kb of the 17q11.2
region upstream of the _NF1_ gene, which is delimited by the sub-duplicons REP-P1/P2 of the proximal NF1-REP P (Figure 3c). The duplication was confirmed by FISH analysis using BAC clone
RP11-753N3 and excluded in the healthy mother (data not shown). Although the father was unavailable for testing, his carrier status could be inferred from the array CGH-assessed presence of
the same duplication in the healthy patient's sister. DISCUSSION RSTS is a genetically heterogeneous disorder with a causative genetic lesion only detected in 50–60% of cases.15, 16, 23
This low mutation rate may be attributable to overlooked mosaic point mutations, incomplete coverage in the sequencing procedure of the regulatory and intronic regions of the known genes
(_CREBBP_ and _EP300_), or the involvement of additional RSTS-causing genes. We addressed this last possibility using array CGH to process a cohort of patients with a definite, probable or
possible clinical diagnosis of RSTS, who were negative to mutation/deletion scans of _CREBBP_ and the FISH analysis of _EP300_, and found that 7 out of 26 patients (27%) bore genomic
imbalances. The rearrangements were _de novo_ in four cases, and were therefore assessed as possible causes of the phenotype. A reassessment of the carriers of the _de novo_ imbalances
confirmed the presence of the main clinical signs of RSTS, such as mental retardation and hand–foot anomalies (Table 2 and Figure 1). To favour the search for positional candidate genes, the
gained or lost genomic regions should be as small as possible. One example is the 500-kb deletion in 7p21.1 in our patient 58: of the two genes located in the deleted region, it is
difficult to link _HDAC9_ to the clinical presentation of RSTS as it encodes a factor (histone deacetylase) that has the opposite role of HAT domain of _CREBBP_ (Figure 2d), but _TWIST1_ may
be relevant (Figure 2d) as it encodes a transcription factor implicated in skeletal development and its mutations lead to SCS, a genetic autosomal dominant condition characterised by
acrocephalosyndactyly and dysmorphisms. _TWIST1_ has been suggested as candidate gene for RSTS,10, 24 on the basis of its nature as a master switch of skeleton morphogenesis and the fact
that, like _CREBBP_, it regulates the development of multiple target genes; however, only a targeted mutational scan of _CREBBP_- and _EP300_-negative patients with a clinical diagnosis of
RSTS can validate its potential role. The conclusion that can be drawn from the described case is that the overlapping features of SCS and RSTS should be considered when clinically
diagnosing the highly different phenotypes of RSTS. As shown in Table 1, patient 58 was given a ‘possible’ clinical RSTS diagnosis because his phenotype mimics RSTS but also shares some of
the signs shown by SCS patients carrying a microdeletion involving the same region or point mutations in the _TWIST1_ gene (Table 2). This ‘borderline’ phenotype confirms the difficulty of
distinguishing the two syndromes in patients with signs of both. A question of differential diagnosis is also raised by patient 51, who carries the large 4.3 Mb 2q22.3q23.1 deletion that
includes the zinc-finger homeobox 1B (_ZEB2_) gene (Figure 2a) and was initially given a diagnosis of ‘probable’ RSTS (Table 1). Point mutations of the _ZEB2_ gene or microdeletions of the
region encompassing it lead to MWS, a genetic autosomal dominant syndrome that is characterised by microcephaly, mental retardation and distinct facial features.25 It seems that
haploinsufficiency of _ZEB2_ and other genes mapping to the same region may explain the phenotype of a patient who has been diagnosed as having RSTS but also has signs overlapping those of
MWS (Table 2). The genomic rearrangements in the remaining two patients carrying _de novo_ imbalances are too gross to identify probable RSTS candidate genes and do not encompass any key
gene associated with a well-defined syndrome (Figure 2). Patient 14 carries a 9-Mb deletion in 2q24.3q31.1 that involves >50 genes, the largest imbalance observed. The very high number of
genes precludes any correlation between the haploinsufficiency of specific dose-sensitive genes and the overall phenotype, but some of the master genes mapping to this gene-rich region
deserve comment. Differently sized deletions (5–33 Mb) that partially overlap that of patient 14 and include the _HOXD_ gene cluster have been described in patients with distinct phenotypes
except for variable limb abnormalities.26, 27, 28, 29 The _HOXD_ genes are members of a homeobox gene family encoding highly conserved transcription factors that have an important
morphogenetic role in all multicell organisms, mainly by acting on the development of the central nervous system, the axial skeleton, the gastrointestinal and urogenital tracts, and limbs,30
and they have been suggested as good candidates for causing the limb anomalies in patients with a 2q24q31 deletion.31 Interestingly, FISH mapping of the distal deletion breakpoint of our
patient showed that it lies outside the _HOXD_ gene cluster, but includes _HOXD13_ and the regulatory elements of the whole cluster (Figure 2b). The absence of the _HOXD_ regulatory elements
may explain why the digital anomalies are much milder than those of patients with a deletion including the _HOXD_ cluster, as it has been previously pointed out in the case of a patient
carrying a similar deletion except for the additional involvement of SNC cluster genes.32 The deletion includes the genes _DLX1_ and _DLX2_ (which encode homeobox transcription factors that
have a role in controlling craniofacial patterning and the differentiation of inhibitory neurons in the forebrain), and their haploinsufficiency may be responsible for our patient's
neurological and dysmorphic phenotype. The deleted region also includes the _ATF2_ gene which, like _CREBBP_,33, 34, 35 encodes a HAT that specifically acetylates histones H2B and H4. The
_de novo_ duplication at 3p13p12.3 observed in patient 61 encompasses a large (5 Mb) but gene-poor region. Among the involved genes, neither the roundabout, axon guidance receptor, homologue
2 (_ROBO2_)36 nor contactin 3 (_CNTN3_) seem to be suitable candidates for RSTS. However, the patient's corpus callosum agenesis and autistic traits may be attributable to the dosage
alteration of the _ROBO2_ gene, which may have a role in axon guidance along the dorsoventral axis in the forebrain37 and could be related to the autistic phenotype.38 The three inherited
imbalances may be of interest because they are all private and none of them is included in the databases of polymorphic variants being yet undescribed in the normal population at a frequency
>1%. Their role in the clinical phenotypes of the carrier patients remains unclear, although reduced penetrance may account for the absence of the putatively associated phenotype in the
healthy parent. The 400-kb dup17q11.2 of patient 29 (Figure 3b) is peculiar because it is embedded in the region deleted in Neurofibromatosis type 1 (NF1) microdeletion syndrome and
localised between the sub-duplicons NF1-REP-P1 and REP-P2. REP-P1 mediates the common NF1 microdeletions with the paralogous NF1-REP-M,39 whereas REP-P2 has not been reported to be involved
in NF1 deletions. This imbalance is the exact reciprocal duplication of a recently reported deletion involving the same region,40 both of which can be considered genomic rearrangements
insofar as they are mediated by non-allelic homologous recombination (NAHR) of REP-P1 and REP-P2. The deletion described by Douglas _et al_40 have been associated with an overgrowth syndrome
that is presumably because of the haploinsufficiency of the _RNF135_ gene, which has been found to be mutated in another group of patients with the same overgrowth syndrome. In our case,
the duplication is inherited and so it is only possible to hypothesise that the gain of dose of the genes localised in the duplicated interval (_RNF135_, _ADAP2_, _C17orf42_, _ATAD5_,
_CRLF3_ and _LRRC37B2_) (Figure 3c) may contribute to the patient's skeletal anomalies (Figure 3a) and mental retardation. In general, the phenotype resulting from duplications that are
even much larger than this is subtle and may be overlooked, as is indicated by the adult age at which a diagnosis was suggested for our patient. In conclusion, this study is the first
systematic array CGH analysis of a cohort of patients with RSTS or RSTS-like features previously screened for mutations in the main _CREBBP_ gene and deletions in regions harbouring the
causative _CREBBP_ and _EP300_ genes. The large proportion of chromosomal rearrangements in regions other than those of _CREBBP_/_EP300_ genes confirms that array CGH is a suitable
diagnostic approach. As no consensus list of diagnostic criteria is yet available for RSTS, array-CGH can be considered a useful means of differentiating patients with overlapping clinical
signs. In relation to its use in identifying novel positional RSTS candidate genes, further studies may detect recurrent imbalances or validate/exclude the role of _TWIST1_ in the aetiology
of RSTS. It is very difficult to interpret the effect of the four _de novo_ chromosome abnormalities found in our patients, and it is not possible to exclude a coincidental finding because
of the extremely broad clinical spectrum of RSTS or a cryptic differential diagnosis. Furthermore, the fact that the microdeletions and microduplication of cases 78, 29 and 45 are inherited
from healthy parents but not listed as CNVs makes it difficult to interpret their contribution to the clinical phenotype. REFERENCES * Rubinstein JH, Taybi H : Broad thumbs and toes and
facial abnormalities. A possible mental retardation syndrome. _Am J Dis Child_ 1963; 105: 588–608. Article CAS Google Scholar * Miller RW, Rubinstein JH : Tumors in Rubinstein-Taybi
syndrome. _Am J Med Genet_ 1995; 56: 112–115. Article CAS Google Scholar * Masuno M, Imaizumi K, Ishii T, Kuroki Y, Baba N, Tanaka Y : Pilomatrixomas in Rubinstein-Taybi syndrome. _Am J
Med Genet_ 1998; 77: 81–82. Article CAS Google Scholar * Schepis C, Greco D, Siragusa M, Batolo D, Romano C : Rubinstein-Taybi syndrome with epidermal nevus: a case report. _Pediatr
Dermatol_ 2001; 18: 34–37. Article CAS Google Scholar * Petrij F, Giles RH, Dauwerse HG _et al_: Rubinstein-Taybi syndrome caused by mutations in the transcriptional coactivator CBP.
_Nature_ 1995; 376: 348–351. Article CAS Google Scholar * Roelfsema JH, White SJ, Ariyürek Y _et al_: Genetic heterogeneity in Rubinstein-Taybi syndrome: mutations in both the CBP and
EP300 genes cause disease. _Am J Hum Genet_ 2005; 76: 572–580. Article CAS Google Scholar * Giordano A, Avantaggiati ML : p300 and CBP: partners for life and death. _J Cell Physiol_ 1999;
181: 218–230. Article CAS Google Scholar * McGaughran JM, Gaunt L, Dore J, Petrij F, Dauwerse HG, Donnai D : Rubinstein-Taybi syndrome with deletions of FISH probe RT1 at 16p13.3: two UK
patients. _J Med Genet_ 1996; 33: 82–83. Article CAS Google Scholar * Bartsch O, Wagner A, Hinkel GK _et al_: FISH studies in 45 patients with Rubinstein-Taybi syndrome: deletions
associated with polysplenia, hypoplastic left heart and death in infancy. _Eur J Hum Genet_ 1999; 7: 748–756. Article CAS Google Scholar * Petrij F, Dauwerse HG, Blough RI _et al_:
Diagnostic analysis of the Rubinstein-Taybi syndrome: five cosmids should be used for microdeletion detection and low number of protein truncating mutations. _J Med Genet_ 2000; 37: 168–176.
Article CAS Google Scholar * Gervasini C, Castronovo P, Bentivegna A _et al_: High frequency of mosaic CREBBP deletions in Rubinstein-Taybi syndrome patients and mapping of somatic and
germ-line breakpoints. _Genomics_ 2007; 90: 567–573. Article CAS Google Scholar * Coupry I, Monnet L, Attia AA, Taine L, Lacombe D, Arveiler B : Analysis of CBP (CREBBP) gene deletions in
Rubinstein-Taybi syndrome patients using real-time quantitative PCR. _Hum Mutat_ 2004; 23: 278–284. Article CAS Google Scholar * Kriek M, Knijnenburg J, White SJ _et al_: Diagnosis of
genetic abnormalities in developmentally delayed patients: a new strategy combining MLPA and array-CGH. _Am J Med Genet A_ 2007; 143: 610–614. Article Google Scholar * Udaka T, Kurosawa K,
Izumi K _et al_: Screening for partial deletions in the CREBBP gene in Rubinstein-Taybi syndrome patients using multiplex PCR/liquid chromatography. _Genet Test_ 2006; 10: 265–271. Article
CAS Google Scholar * Bartsch O, Schmidt S, Richter M _et al_: DNA sequencing of CREBBP demonstrates mutations in 56% of patients with Rubinstein-Taybi syndrome (RSTS) and in another
patient with incomplete RSTS. _Hum Genet_ 2005; 117: 485–493. Article CAS Google Scholar * Bentivegna A, Milani D, Gervasini C _et al_: Rubinstein-Taybi syndrome: spectrum of CREBBP
mutations in Italian patients. _BMC Med Genet_ 2006; 7: 77. Article Google Scholar * Roelfsema JH, Peters DJ : Rubinstein-Taybi syndrome: clinical and molecular overview. _Expert Rev Mol
Med_ 2007; 9: 1–16. Article Google Scholar * Bartholdi D, Roelfsema JH, Papadia F _et al_: Genetic heterogeneity in Rubinstein-Taybi syndrome: delineation of the phenotype of the first
patients carrying mutations in EP300. _J Med Genet_ 2007; 44: 327–333. Article CAS Google Scholar * Zimmermann N, Acosta AM, Kohlhase J, Bartsch O : Confirmation of EP300 gene mutations
as a rare cause of Rubinstein-Taybi syndrome. _Eur J Hum Genet_ 2007; 15: 837–842. Article CAS Google Scholar * Foley P, Bunyan D, Stratton J, Dillon M, Lynch SA : Further case of
Rubinstein-Taybi syndrome due to a deletion in EP300. _Am J Med Genet A_ 2009; 149A: 997–1000. Article CAS Google Scholar * Stef M, Simon D, Burgelin I _et al_: Testing and improving
experimental parameters for the use of low molecular weight targets in array-CGH experiments. _Hum Mutat_ 2006; 27: 1143–1150. Article CAS Google Scholar * Lichter P, Cremer T :
Chromosome analysis by non-isotopic _in situ_ hybridization; in Rooney DE, Czepulkowski BH (eds):: _Human Cytogenetics: A Practical Approach_, 2nd edn. Oxford: IRL, 1992, Vol 1, pp 157–192.
Google Scholar * Coupry I, Roudaut C, Stef M _et al_: Molecular analysis of the CBP gene in 60 patients with Rubinstein-Taybi syndrome. _J Med Genet_ 2002; 39: 415–421. Article CAS Google
Scholar * Lowry RB : Overlap between Rubinstein-Taybi and Saethre-Chotzen syndromes: a case report. _Am J Med Genet Suppl_ 1990; 6: 73–76. CAS PubMed Google Scholar * Garavelli L,
Zollino M, Mainardi PC _et al_: Mowat-Wilson syndrome: facial phenotype changing with age: study of 19 Italian patients and review of the literature. _Am J Med Genet A_ 2009; 149A: 417–426.
Article CAS Google Scholar * Nixon J, Oldridge M, Wilkie AO, Smith K : Interstitial deletion of 2q associated with craniosynostosis, ocular coloboma, and limb abnormalities: cytogenetic
and molecular investigation. _Am J Med Genet_ 1997; 70: 324–327. Article CAS Google Scholar * Slavotinek A, Schwarz C, Getty JF, Stecko O, Goodman F, Kingston H : Two cases with
interstitial deletions of chromosome 2 and sex reversal in one. _Am J Med Genet_ 1999; 86: 75–81. Article CAS Google Scholar * Bijlsma EK, Knegt AC, Bilardo CM _et al_: Increased nuchal
translucency and split-hand/foot malformation in a fetus with an interstitial deletion of chromosome 2q that removes the SHFM5 locus. _Prenat Diagn_ 2005; 25: 39–44. Article CAS Google
Scholar * Svensson AM, Curry CJ, South ST _et al_: Detection of a _de novo_ interstitial 2q microdeletion by CGH microarray analysis in a patient with limb malformations, microcephaly and
mental retardation. _Am J Med Genet A_ 2007; 143A: 1348–1353. Article CAS Google Scholar * Goodman FR : Limb malformations and the human HOX genes. _Am J Med Genet_ 2002; 112: 256–265.
Article Google Scholar * Del Campo M, Jones MC, Veraksa AN _et al_: Monodactylous limbs and abnormal genitalia are associated with hemizygosity for the human 2q31 region that includes the
HOXD cluster. _Am J Hum Genet_ 1999; 65: 104–110. Article CAS Google Scholar * Pescucci C, Caselli R, Grosso S _et al_: 2q24-q31 deletion: report of a case and review of the literature.
_Eur J Med Genet_ 2007; 50: 21–32. Article CAS Google Scholar * Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y : The transcriptional coactivators p300 and CBP are histone
acetyltransferases. _Cell_ 1996; 87: 953–959. Article CAS Google Scholar * Schiltz RL, Mizzen CA, Vassilev A, Cook RG, Allis CD, Nakatani Y : Overlapping but distinct patterns of histone
acetylation by the human coactivators p300 and PCAF within nucleosomal substrates. _J Biol Chem_ 1999; 274: 1189–1192. Article CAS Google Scholar * Kalkhoven E : CBP and p300: HATs for
different occasions. _Biochem Pharmacol_ 2004; 68: 1145–1155. Article CAS Google Scholar * Lu W, van Eerde AM, Fan X _et al_: Disruption of ROBO2 is associated with urinary tract
anomalies and confers risk of vesicoureteral reflux. _Am J Hum Genet_ 2007; 80: 616–632. Article CAS Google Scholar * Devine CA, Key B : Robo-Slit interactions regulate longitudinal axon
pathfinding in the embryonic vertebrate brain. _Dev Biol_ 2008; 313: 371–383. Article CAS Google Scholar * Anitha A, Nakamura K, Yamada K _et al_: Genetic analyses of roundabout (ROBO)
axon guidance receptors in autism. _Am J Med Genet B Neuropsychiatr Genet_ 2008; 147B: 1019–1027. Article CAS Google Scholar * Dorschner MO, Sybert VP, Weaver M, Pletcher BA, Stephens K :
NF1 microdeletion breakpoints are clustered at flanking repetitive sequences. _Hum Mol Genet_ 2000; 9: 35–46. Article CAS Google Scholar * Douglas J, Cilliers D, Coleman K _et al_:
Mutations in RNF135, a gene within the NF1 microdeletion region, cause phenotypic abnormalities including overgrowth. _Nat Genet_ 2007; 39: 963–965. Article CAS Google Scholar Download
references ACKNOWLEDGEMENTS We would like thank the patients’ families for participating in this study, and the clinicians who provided some of the patients (Professor G Cocchi, Centro per
lo Studio delle Malformazioni Congenite, Istituto Clinico di Pediatria preventiva e Neonatologia, University of Bologna, and Professor ML Giovannucci-Uzielli, Unità di Genetica, Dip
Pediatria, Osp Meyer, University of Florence). This study was supported by 2007–2008 grant from the Associazione Studio Malformazioni (ASM) to LL (University of Milan) and AS (Fondazione
Ospedale Maggiore, Policlinico, Regina Elena, Mangiagalli). We would also like to thank the Galliera Genetic Bank Italian Telethon project GTF4003 for providing us with lymphoblastoid
cellular lines of some of the studied patients. We gratefully acknowledge our feedback discussion with the group of Dr Angel Barco (Universidad Miguel Hernandez De Elche, Alicante, Spain) in
the framework of the Italy–Spain bilateral MIUR project on RSTS (IT08143C4F). AUTHOR INFORMATION Author notes * Cristina Gervasini and Federica Mottadelli: These authors contributed equally
to this work. AUTHORS AND AFFILIATIONS * Division of Medical Genetics, San Paolo School of Medicine, University of Milan, Milan, Italy Cristina Gervasini, Federica Mottadelli, Paola
Castronovo & Lidia Larizza * Sezione di Biologia Generale e Genetica Medica, Università di Pavia, Italy Roberto Ciccone & Orsetta Zuffardi * I Clinica Pediatrica, Fondazione
Policlinico Mangiagalli Regina Elena, Milan, Italy Donatella Milani & Angelo Selicorni * UO Genetica Medica, AO ‘G Rummo’, Benevento, Italy Gioacchino Scarano * Department of Obstetrics
and Pediatrics, Clinical Genetic Unit, Fondazione Ospedale Maggiore Policlinico Mangiagalli e Regina Elena, Milan, Italy Maria Francesca Bedeschi * Consultorio Genetico APSS, Trento, Italy
Serena Belli * Auxoendocrinologia, Ospedale Pediatrico, Brescia, Italy Alba Pilotta Authors * Cristina Gervasini View author publications You can also search for this author inPubMed Google
Scholar * Federica Mottadelli View author publications You can also search for this author inPubMed Google Scholar * Roberto Ciccone View author publications You can also search for this
author inPubMed Google Scholar * Paola Castronovo View author publications You can also search for this author inPubMed Google Scholar * Donatella Milani View author publications You can
also search for this author inPubMed Google Scholar * Gioacchino Scarano View author publications You can also search for this author inPubMed Google Scholar * Maria Francesca Bedeschi View
author publications You can also search for this author inPubMed Google Scholar * Serena Belli View author publications You can also search for this author inPubMed Google Scholar * Alba
Pilotta View author publications You can also search for this author inPubMed Google Scholar * Angelo Selicorni View author publications You can also search for this author inPubMed Google
Scholar * Orsetta Zuffardi View author publications You can also search for this author inPubMed Google Scholar * Lidia Larizza View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Lidia Larizza. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gervasini, C., Mottadelli, F., Ciccone, R. _et al._ High frequency of copy number imbalances in Rubinstein–Taybi patients
negative to _CREBBP_ mutational analysis. _Eur J Hum Genet_ 18, 768–775 (2010). https://doi.org/10.1038/ejhg.2010.1 Download citation * Received: 07 May 2009 * Revised: 08 September 2009 *
Accepted: 09 December 2009 * Published: 03 February 2010 * Issue Date: July 2010 * DOI: https://doi.org/10.1038/ejhg.2010.1 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative KEYWORDS * Rubinstein–Taybi syndrome * array-CGH * genomic imbalances * differential diagnosis * genotype-phenotype correlations
