Correlation between microrna-34a levels and lens opacity severity in age-related cataracts
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT PURPOSE MicroRNA 34a (miR-34a) is involved in regulating tissue senescence. However, the role of miR-34a in age-related cataracts is unclear. In this study, we evaluated the
correlations among the severity of lens opacity, patient age, and miR-34a expression level in the lens epithelium of age-related cataracts for clarifying the role of miR-34a in the lens
senescence. METHODS This study was carried as a case control study in the Department of Ophthalmology, Taipei Veterans General Hospital, Taiwan. We recorded age of each patient at the time
of their cataract surgery and information regarding lens opacity according to a modified version of the Lens Opacities Classification System III. Correlations among age, lens opacity, and
miR-34a expression levels were evaluated. RESULTS This study evaluated 110 patients with a mean age of 73.19 years (SD±10.2). Older patients had higher nuclear cataract (NC), cortical (C),
and posterior subcapsular cataract (P) scores (one-way analysis of variance (ANOVA), _P_<0.05). miR-34a expression levels were significantly different between each age group (ANOVA _post
hoc_ Bonferroni’s test, _P_<0.001), and there were moderate correlations between high NC, C, and P cataract scores and high miR-34a levels (Pearson correlation coefficient; _R_=0.606,
0.575, and 0.515, respectively). CONCLUSIONS The current study demonstrated positive correlations between high miR-34a levels and high lens opacity severity in NC, C, or P cataracts. These
results suggest that miR-34a expression has a role in lens senescence. SIMILAR CONTENT BEING VIEWED BY OTHERS LNCRNA GAS5 REGULATES MIGRATION AND EPITHELIAL-TO-MESENCHYMAL TRANSITION IN LENS
EPITHELIAL CELLS VIA THE MIR-204-3P/_TGFBR1_ AXIS Article 16 December 2021 MIR-204-5P MAY REGULATE OXIDATIVE STRESS IN MYOPIA Article Open access 29 April 2024 URIC ACID–DRIVEN NLRP3
INFLAMMASOME ACTIVATION TRIGGERS LENS EPITHELIAL CELL SENESCENCE AND CATARACT FORMATION Article Open access 09 March 2024 INTRODUCTION Studies have provided many evidences of the
participation of microRNAs in multiple cellular functions, such as cell proliferation,1 cell apoptosis,2 stress response,3 and senescence,4 mainly through post-transcriptional mRNA silencing
and subsequent protein translation inhibition. MicroRNAs, which are groups of endogenous and small noncoding RNAs, have also been found to be biological modulators at the post-translational
level through complementary or partial complementary binding with the 3′ untranslated region (UTR) of target mRNA.5, 6 MicroRNAs are also involved in DNA damage response-induced senescence,
which is mainly governed by the p53 pathway.7 The microRNA-34a (miR-34a), the most apparent of the p53-induced microRNAs, has a role in senescence through the upregulation of acetylated p53
and activation of apoptosis in a positive feedback loop.2 However, very less information is available regarding miR-34a in ocular aging processes, such as cataracts. Cataracts, the most
common cause of blindness worldwide,8 are significantly related to the aging process. Currently, many factors such as diabetes mellitus, ultraviolet exposure, systemic drugs, and other
ocular diseases are known to be related to cataract formation. Among these disorders, oxidative stress with the subsequent formation of reactive oxygen species reactive oxygen species (ROS)
is thought to be a major predisposing factor in age-related cataracts.9 Recently, a biological factor, silent information regulator T1 (SirT1), was proposed as an element involved in
delaying the aging process and lifespan extension by suppressing NF-_κ_B signaling. We have found that decreased expression of the _SirT1_ gene is associated with higher cataract severity
and patient age in our prior study,10 but miR-34a expression in age-related cataracts has not been understood well. The aim of this study was to investigate the expression of miR-34a in the
lens epithelium of patients with age-related cataracts. To further evaluate the correlations among patient age, cataract severity, and miR-34a expression, the Lens Opacities Classification
System III (LOC III)11 was conducted to stratify the patients according to cataract type and severity. MATERIALS AND METHODS Lens epithelium samples were obtained from 110 eyes between
January 2008 and December 2010. This study followed the tenets of the Declaration of Helsinki. All patients underwent a complete preoperative ophthalmologic examination and only those who
had no ocular diseases other than age-related cataracts were included. Patients who had previously undergone ocular surgery or those with diabetes were not included from this study. All
samples were collected after obtaining informed consent from the patient. Lens epithelium samples were obtained by intact continuous curvilinear capsulorhexis, with care taken to avoid
vascular contact or damage to the iris or other intraocular structures. Cataract type and severity were graded and recorded on the basis of a modified version of the LOCSIII by using six
silt-lamp images for grading nuclear color and nuclear retro-illumination images for grading posterior subcapsular (P) cataracts. Each scale on the LOCSIII is a decimalized scale ranging
from 0.1 (a completely clear or colorless lens) to 5.9 (upper value on the C and P scales indicating complete opacification of the cortex or posterior capsule) and 6.9 on the NC scales
(indicating advanced opacification and brunescence of the nucleus). All LOCSIII scorings among subjects was carried out and consisted up to at least three ophthalmologists. The control lens
epithelium samples with a LOCSIII score of C1, NC1, and P1 were obtained from the vitrectomy operation for epiretinal membranes (_N_=13). MICRORNA ISOLATION AND MICRORNA QUANTITATIVE
REAL-TIME REVERSE TRANSCRIPTASE (RT-PCR) The expression levels of miR-34a microRNA were determined by quantitative real-time RT-PCR (qRT-PCR) as previously described.12 Briefly, a mirVana
PARIS kit (Ambion, Grand Island, NY, USA) was used to isolate small RNAs from total RNA according to the manufacturer’s instructions. The mature miR-34a sequence is (5′–3′)
UGGCAGUGUCUUAGCUGGUUGUU. For microRNA quantitation, qRT-PCR was performed using TaqMan miRNA assays (Applied Biosystems, Grand Island, NY, USA) with specific primer sets. All reagents and
protocols were obtained from (Applied Biosystems) and detection was performed using a 7900HT fast real-time PCR system (Applied Biosystems) with RNU6B as an internal control. The conditions
for amplification were as follows: one cycle of 94 °C for 2 min, followed by 50 cycles of 94 °C for 20 s, 57 °C for 20 s, and 70 °C for 20 s. MicroRNA-specific qRT-PCR was performed in
triplicate and repeated three times, the expression ratio of miR-34a/RUN6B in control patients was defined as 1. STATISTICAL METHODS For statistical analysis, patient age was expressed as
mean±SD and miR-34a mRNA levels were expressed as mean±SE. The correlations among LOCSIII score, age, and levels of miR-34a mRNA were analyzed using one-way analysis of variance (ANOVA),
followed by _post hoc_ Bonferroni’s test. The correlation between LOCSIII score and age, and LOCSIII score and levels of miR-34a mRNA expression were analyzed using the Pearson correlation
coefficient and unpaired Student’s _t_-test. A _P_-value of <0.05 was considered statistically significant. All analyses were performed using SPSS 12.0 (IBM, Armonk, NY, USA). RESULTS At
the end of enrollment, 110 patients were included in this study. Patient ages ranged from 55–92 years with an average of 73.19 years (SD 10.2). We further stratified patients according to
age into four groups: between 55 and 64 years (25; 22.7%), between 65 and 74 years (35; 31.8%), between 75 and 84 years (28; 25.5%), and older than 85 years (22; 20%). Considering the entire
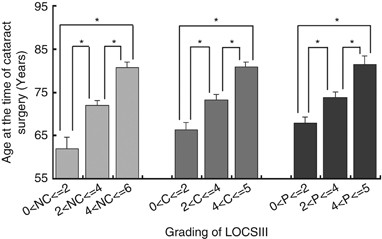
patient cohort, the age at the time of cataract surgery showed significant differences with respect to the grading of nuclear cataracts (NCs) (one-way ANOVA, _P_<0.05) (Figure 1). In
addition, the age at the time of cataract surgery showed significant differences with respect to the grading of cortical cataracts (C) (one-way ANOVA, _P_<0.05) and the grading of P
cataract (one-way ANOVA, _P_<0.05) (Figure 1). There were correlations among older ages at the time of cataract surgery and higher NC, C, and P LOCSIII scores (Pearson correlation
coefficient; _R_=0.399; _P_<0.001; _R_=0.479; _P_<0.001; _R_=0.410; _P_<0.001, respectively). We compared miR-34a levels between each age group and found that the older the patient,
the greater the miR-34a level. The mean miR-34a level of all patients was 1.18 (SE±0.01) and the mean miR-34a levels in patients older than 85 years (mean±SE, 1.30±0.03) was significantly
different from those in patients whose age was between 75 and 84 years (mean±SE, 1.24±0.03) (ANOVA _post hoc_ Bonferroni’s test, _P_<0.001). In addition, the mean miR-34a levels of the
patients between the age of 75 and 84 years were significantly different from those of the patients aged between 65 and 74 years (mean±SE, 1.15±0.02) (ANOVA _post hoc_ Bonferroni’s test,
_P_<0.001). There was also a significant difference between the mean miR-34a levels of patients aged 65–74 years and those of patients aged 55–64 years (mean±SE, 1.03±0.02) (ANOVA _post
hoc_ Bonferroni’s test, _P_<0.001) (data not shown). A scatter plot showed a moderate correlation between age at the time of cataract surgery and miR-34a levels in the lens epithelium
(Pearson correlation coefficient _R_=0.683; _P_<0.001, Figure 2). In NC cataracts, there was a moderate correlation between miR-34a levels and lens opacity severity (Pearson correlation
coefficient _R_=0.606; _P_<0.001, Figure 3). Moreover, there were moderate correlations between miR-34a levels and severity of C cataracts (Pearson correlation coefficient _R_=0.575;
_P_<0.001) and between miR-34a levels and P cataract severity (Pearson correlation coefficient _R_=0.518; _P_<0.001). To further examine relations among levels of miR-34a expression
levels, age of patients, and different levels of cataracts, we stratified patients into nine subgroups according to their levels of cataracts. It was noted that in each subgroup, miR-34a
expression levels increased as older age of patients (Figure 4a). Then, in each subgroup, we compared miR-34a expression levels between samples from male and female subjects (Figure 4b).
There was no significant difference between different gender samples in any subgroup of our study. DISCUSSION Cataract, the first leading cause of blindness in the world, accounts for 47.8%
of blindness that equals 37 millions blind people in the world.8 Notably, 90% of cataract-related blindness are in the developing countries.8 Most cataracts are age-related; however, there
are several risk factors about cataractogenesis.13, 14 Previous studies focused on prevalence differences of three specific types of lens opacity: nuclear sclerosis, C cataract, and P
cataract. Owing to variation of subject race and age, nuclear sclerosis is most commonly noted in Canadian, Chinese, and American whites,15, 16, 17 while African, Japanese, and Singaporean
present higher prevalence of C cataract than nuclear sclerosis.16 However, there is scant information about certain factors among different types of lens opacity. Our study enrolled Chinese
patients aged 55 and older with age-related cataract, and analyzed detailed information among individual grades of cataracts according to LOC III. We found that cataract in all subtypes of
cataracts got more severe and higher miR-34a expression levels as subjects aged older. Gender is proposed as a risk factor to cataract formation;13 however, we could not find such results
from our study (Figure 4b). We believed a larger scale of further study on miR-34a may be needed to answer the question. Some confirmed risk factors to cataract formation are related to
oxidative stress, such as chronic ultraviolet light exposure, smoking, and diabetes.14, 18 Oxidative stress is proved to cause DNA damage and subsequent cataract formation.19, 20 Blue
Mountain Eye Study proved antioxidants intake, including beta-carotene, zinc, and vitamins A, C, and E, reduced nuclear sclerosis cataract formation.21 Sorte _et al_20 examined DNA damage
amounts in lens epithelial cells right after cataract surgery and found that DNA damage products were noted maximally in C cataract subject samples. Those studies disclosed that oxidative
stress prevention may be related to cataract prevention. Recently, one study on _Caenorhabditis elegans_ with miR-34 loss-of-function mutation showed that subjects extend their lifespan, and
increases resistance to oxidative stress.22 Taken together, we supposed that miR-34a may have their role in age-related catracts and from our study, we proposed that miR-34a is correlated
with age of patients (Figure 2) and severity of cataracts (Figure 4a). MiR-34a is a microRNA that regulates biological processes through complementary binding to its target at the 3′ UTR
site.23 MiR-34a also interferes with the cell cycle and proptosis via the p53 pathway in cancer cells.24, 25, 26 MiR-34a was recently reported to be involved in senescence. Upregulation of
miR-34a was observed in a premature senescence model induced by hydrogen peroxide27 and, alternatively, antisense inhibition of miR-34a hindered the onset of replicative senescence.28 Zhao
_et al_12 studied bone-marrow-derived endothelial progenitor cells (EPCs) from rats and found that overexpression of miR-34a led to the inhibition of EPC-mediated angiogenesis and induction
of senescence by suppressing SirT1. In addition, Ito _et al_29 found that miR-34a increased with age in endothelial cells in senescent human umbilical cord vein endothelial cells and in the
hearts and spleens of older mice. However, there is no study yet about correlation between miR-34a expression and ocular senescent tissues. Our current study focused on miR-34a expression in
the lens epithelium of age-related cataracts for investigating their role in the ocular aging process. There were significant correlations among NC, C, and P cataracts and age at the time
of surgery (Figure 1). We also investigated the association between miR-34a expression in the lens epithelium and cataract subtypes and severity, which was classified according to LOCSIII.
Our results show that the level of miR-34a expression in the lens epithelium increased with aging (Figure 2) and with the increase in the severity of NC, C, and P cataracts. (Figure 3) We
believe that this is the first report describing a role for miR-34a in the senescence of aging lenses. Age-related cataract formation involves several mechanisms, including ROS
accumulation.9 UV exposure and cigarette smoking are two examples of ROS-generating stress resources. Lou _et al_30 used hydrogen peroxide exposure to generate ROS in rat lenses and found
that most of the ROS accumulated in the lens epithelium and this resulted in a cataract. Grape seed proanthocyanidin extract is known to protect lens epithelial cells from ROS damage31 in a
dose-dependent manner and it exerts its effects via nuclear SirT1 expression.32 Recently, microRNAs, such as let-7 members, have been shown to have a role in newt lens regeneration.33, 34
MiR-34a participates in the aging process by suppression of SirT1 expression.2, 35 Ito _et al_29 demonstrated miR-34a regulates endothelial senescene directly through SirT1 that
overexpression of miR-34a can reduce SirT1 expression and increase senescene; conversely, SirT1 expression will increase if miR-34a is knocked down. From our prior study,10 we found that
decreased expression of SirT1 correlated with the severity of age-related cataracts, age at the time of surgery, and with NC, C, and P subtypes. In this study, we found that miR-34a
expression positively correlated with the cataract severity according to LOCSIII classification (Figure 1). In our current study, our results support the hypothesis that miR-34a expression
increases with aging (_R_=0. 683; Figure 2). This result implies that miR-34a expression in the ocular aging process occurs in parallel with the aging process of the entire body through
miR-34a–SirT1 pathway. We also stratified the age-related cataracts into different subtypes according to LOCSIII and found that miR-34a positively correlated with the severity of all
subtypes (Figure 3). Ito _et al_29 used population doubling time as a time course to follow miR-34a expression, whereas our study showed that the LOCSIII system correlated with miR-34a
expression in aging human lenses. In conclusion, in order to understand miR-34a expression specifically in human age-related cataracts, we evaluated miR-34a expression in lens epithelial
cells obtained via cataract surgeries. We discovered that miR-34a expression not only correlated with age at the time of surgery but also correlated with the different severity of aging
cataracts in nuclear, C, or P subtypes. Given these results, it is apparent that miR-34a expression has a role in lens senescence and may be a target for scavengers of oxidative stresses in
the human ocular aging process. REFERENCES * Zhao C, Sun G, Li S, Lang MF, Yang S, Li W _et al_. MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting
nuclear receptor TLX signaling. _Proc Natl Acad Sci USA_ 2010; 107: 1876–1881. Article CAS PubMed PubMed Central Google Scholar * Yamakuchi M, Ferlito M, Lowenstein CJ . miR-34a
repression of SIRT1 regulates apoptosis. _Proc Natl Acad Sci USA_ 2008; 105: 13421–13426. Article CAS PubMed PubMed Central Google Scholar * Leung AK, Sharp PA . MicroRNA functions in
stress responses. _Mol Cell_ 2010; 40: 205–215. Article CAS PubMed PubMed Central Google Scholar * Tazawa H, Tsuchiya N, Izumiya M, Nakagama H . Tumor-suppressive miR-34a induces
senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. _Proc Natl Acad Sci USA_ 2007; 104: 15472–15477. Article CAS PubMed PubMed Central Google
Scholar * Bartel DP . MicroRNAs: genomics, biogenesis, mechanism, and function. _Cell_ 2004; 116: 281–297. Article CAS PubMed Google Scholar * Bartel DP . MicroRNAs: target recognition
and regulatory functions. _Cell_ 2009; 136: 215–233. Article CAS PubMed PubMed Central Google Scholar * Chen LH, Chiou GY, Chen YW, Li HY, Chiou SH . MicroRNA and aging: a novel
modulator in regulating the aging network. _Ageing Res Rev_ 9 (Suppl 1): S59–S66. Article CAS PubMed Google Scholar * Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram
R, Pokharel GP _et al_. Global data on visual impairment in the year 2002. _Bull World Health Organ_ 2004; 82: 844–851. PubMed PubMed Central Google Scholar * Truscott RJ . Age-related
nuclear cataract-oxidation is the key. _Exp Eye Res_ 2005; 80: 709–725. Article CAS PubMed Google Scholar * Lin TJ, Peng CH, Chiou SH, Liu JH, Lin Chung W, Tsai CY _et al_. Severity of
lens opacity, age, and correlation of the level of silent information regulator T1 expression in age-related cataract. _J Cataract Refract Surg_ 37: 1270–1274. Article Google Scholar *
Chylack Jr LT, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL _et al_. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. _Arch Ophthalmol_
1993; 111: 831–836. Article PubMed Google Scholar * Zhao T, Li J, Chen AF . MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing
silent information regulator 1. _Am J Physiol Endocrinol Metab_ 2010; 299: E110–E116. Article CAS PubMed PubMed Central Google Scholar * Taylor HR . Epidemiology of age-related
cataract. _Eye (Lond)_ 1999; 13 (Pt 3b): 445–448. Article Google Scholar * West S . Epidemiology of cataract: accomplishments over 25 years and future directions. _Ophthalmic Epidemiol_
2007; 14: 173–178. Article PubMed Google Scholar * Tsai SY, Hsu WM, Cheng CY, Liu JH, Chou P . Epidemiologic study of age-related cataracts among an elderly Chinese population in
Shih-Pai, Taiwan. _Ophthalmology_ 2003; 110: 1089–1095. Article PubMed Google Scholar * Abraham AG, Condon NG, West Gower E . The new epidemiology of cataract. _Ophthalmol Clin North Am_
2006; 19: 415–425. PubMed Google Scholar * Machan CM, Hrynchak PK, Irving EL . Modeling the prevalence of age-related cataract: Waterloo Eye Study. _Optom Vis Sci_ 2011; 89 (2): 130–136.
Article Google Scholar * Agte VV, Tarwadi KV . Combination of diabetes and cataract worsens the oxidative stress and micronutrient status in Indians. _Nutrition_ 2008; 24: 617–624. Article
CAS PubMed Google Scholar * Ates O, Alp HH, Kocer I, Baykal O, Salman IA . Oxidative DNA damage in patients with cataract. _Acta Ophthalmol_ 2010; 88: 891–895. Article CAS PubMed
Google Scholar * Sorte K, Sune P, Bhake A, Shivkumar VB, Gangane N, Basak A . Quantitative assessment of DNA damage directly in lens epithelial cells from senile cataract patients. _Mol
Vis_ 2011; 17: 1–6. CAS PubMed PubMed Central Google Scholar * Tan AG, Mitchell P, Flood VM, Burlutsky G, Rochtchina E, Cumming RG _et al_. Antioxidant nutrient intake and the long-term
incidence of age-related cataract: the Blue Mountains Eye Study. _Am J Clin Nutr_ 2008; 87: 1899–1905. Article CAS PubMed Google Scholar * Yang J, Chen D, He Y, Melendez A, Feng Z, Hong
Q _et al_. MiR-34 modulates _Caenorhabditis elegans_ lifespan via repressing the autophagy gene atg9. _Age (Dordr)_ 2013; 35 (1): 11–22. Article Google Scholar * Ambros V . MicroRNAs: tiny
regulators with great potential. _Cell_ 2001; 107: 823–826. Article CAS PubMed Google Scholar * Christoffersen NR, Shalgi R, Frankel LB, Leucci E, Lees M, Klausen M _et al_.
p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC. _Cell Death Differ_ 17: 236–245. Article Google Scholar * Mraz M, Pospisilova S, Malinova K,
Slapak I, Mayer J . MicroRNAs in chronic lymphocytic leukemia pathogenesis and disease subtypes. _Leuk Lymphoma_ 2009; 50: 506–509. Article CAS PubMed Google Scholar * Chen QR, Yu LR,
Tsang P, Wei JS, Song YK, Cheuk A _et al_. Systematic proteome analysis identifies transcription factor YY1 as a direct target of miR-34a. _J Proteome Res_ 2011; 10: 479–487. Article CAS
PubMed Google Scholar * Maes OC, Sarojini H, Wang E . Stepwise up-regulation of microRNA expression levels from replicating to reversible and irreversible growth arrest states in WI-38
human fibroblasts. _J Cell Physiol_ 2009; 221: 109–119. Article CAS PubMed Google Scholar * Fujita K, Mondal AM, Horikawa I, Nguyen GH, Kumamoto K, Sohn JJ _et al_. p53 isoforms
Delta133p53 and p53beta are endogenous regulators of replicative cellular senescence. _Nat Cell Biol_ 2009; 11: 1135–1142. Article CAS PubMed PubMed Central Google Scholar * Ito T, Yagi
S, Yamakuchi M . MicroRNA-34a regulation of endothelial senescence. _Biochem Biophys Res Commun_ 398: 735–740. Article CAS PubMed Google Scholar * Lou MF, Xu GT, Cui XL . Further
studies on the dynamic changes of glutathione and protein-thiol mixed disulfides in H2O2 induced cataract in rat lenses: distributions and effect of aging. _Curr Eye Res_ 1995; 14: 951–958.
Article CAS PubMed Google Scholar * Barden CA, Chandler HL, Lu P, Bomser JA, Colitz CM . Effect of grape polyphenols on oxidative stress in canine lens epithelial cells. _Am J Vet Res_
2008; 69: 94–100. Article CAS PubMed Google Scholar * Lee YA, Cho EJ, Yokozawa T . Protective effect of persimmon (Diospyros kaki) peel proanthocyanidin against oxidative damage under
H2O2-induced cellular senescence. _Biol Pharm Bull_ 2008; 31: 1265–1269. Article CAS PubMed Google Scholar * Tsonis PA, Call MK, Grogg MW, Sartor MA, Taylor RR, Forge A _et al_.
MicroRNAs and regeneration: Let-7 members as potential regulators of dedifferentiation in lens and inner ear hair cell regeneration of the adult newt. _Biochem Biophys Res Commun_ 2007; 362:
940–945. Article CAS PubMed PubMed Central Google Scholar * Nakamura K, Maki N, Trinh A, Trask HW, Gui J, Tomlinson CR _et al_. miRNAs in newt lens regeneration: specific control of
proliferation and evidence for miRNA networking. _PLoS One_ 5: e12058. Article PubMed PubMed Central Google Scholar * Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH
_et al_. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. _Mol Cell_ 2007; 26: 745–752. Article CAS PubMed PubMed Central Google Scholar
Download references ACKNOWLEDGEMENTS This work was assisted in part by the Division of Experimental Surgery of the Department of Surgery, Taipei Veterans General Hospital. This study was
supported by the National Science Council (98-2314-B-341-001-MY3, 99-2314-B-075-005-MY3 and NSC 101-2314-B-341 -003 -MY3), Taipei Veterans General Hospital (C1-099/ER3-001), Taipei City
Hospital (96/97/98/099XDAA00102/10001-62-042), Shin-Kong Wu Ho-Su Memorial Hospital (SKH-8302-97-DR-25 and SKH-8302-98-DR-26), Zuoying Armed Forces General Hospital (9502/9696/9719),
Cheng-Hsin General Hospital (CHGH-100-01, 100-19, 100-20, 100-22, and 100-40) and Yen-Tjing Ling Medical Foundation (96/97/98), Taiwan. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Ophthalmology, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan K-H Chien, C-M Liang & J-T Chen * School of Medicine, National Yang-Ming
University, Taipei, Taiwan S-J Chen, J-H Liu, L-C Woung, S-H Chiou & C-H Peng * Department of Ophthalmology, Taipei Veterans General Hospital, Taipei, Taiwan S-J Chen & S-H Chiou *
Department of Ophthalmology, Cheng-Hsin General Hospital, Taipei, Taiwan J-H Liu * Department of Optics and Photonics, National Central University, Chung-Li, Taiwan H-M Chang * Department of
Ophthalmology, Taipei City Hospital, Taipei, Taiwan L-C Woung * The Graduate Institute of Clinical Medical Sciences, Chang Gung University College of Medicine; Kaohsiung, T-J Lin *
Kaohsiung, Kaohsiung, Taiwan T-J Lin * Department of Ophthalmology, Zuoying Armed Forces General Hospital, Kaohsiung, Taiwan T-J Lin * Institute of Clinical Medicine, National Yang-Ming
University, Taipei, Taiwan S-H Chiou & C-H Peng * Department of Medical Research and Education, Taipei Veterans General Hospital, Taipei, Taiwan S-H Chiou * Department of Ophthalmology,
Shin–Kong Wu Ho-Su Memorial Hospital and Fu-Jen Catholic University, Taipei, Taiwan C-H Peng Authors * K-H Chien View author publications You can also search for this author inPubMed Google
Scholar * S-J Chen View author publications You can also search for this author inPubMed Google Scholar * J-H Liu View author publications You can also search for this author inPubMed Google
Scholar * H-M Chang View author publications You can also search for this author inPubMed Google Scholar * L-C Woung View author publications You can also search for this author inPubMed
Google Scholar * C-M Liang View author publications You can also search for this author inPubMed Google Scholar * J-T Chen View author publications You can also search for this author
inPubMed Google Scholar * T-J Lin View author publications You can also search for this author inPubMed Google Scholar * S-H Chiou View author publications You can also search for this
author inPubMed Google Scholar * C-H Peng View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to C-H Peng. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION This paper is not presented at any conference. RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chien, KH., Chen, SJ., Liu, JH. _et al._ Correlation between microRNA-34a levels and lens opacity severity in age-related cataracts. _Eye_
27, 883–888 (2013). https://doi.org/10.1038/eye.2013.90 Download citation * Received: 28 June 2012 * Accepted: 20 March 2013 * Published: 10 May 2013 * Issue Date: July 2013 * DOI:
https://doi.org/10.1038/eye.2013.90 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * aging * cataract * microRNA * senescence
