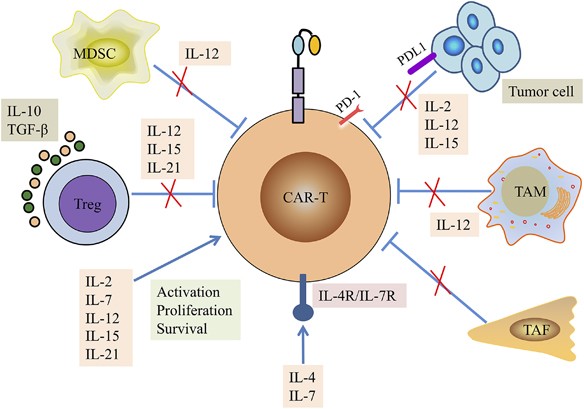
Interleukin-armed chimeric antigen receptor-modified t cells for cancer immunotherapy
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Chimeric antigen receptor-modified T cells (CAR-T) are endowed with cytotoxic specificity to tumor cells. Although CAR-T-based cancer immunotherapy presents curable therapeutic
potential for hematological malignancies, achieving substantial efficacy for solid tumors remain challenging. Researchers have exploited many strategies to enhance the anti-tumor efficacy of
CAR-T cells for solid tumors, among which cytokine-armed CAR-T cells improve the proliferation, survival, homing and other properties of CAR-T cells. Interleukins (ILs), pivotal cytokines
that affect the function of immune cells, were co-expressed in CAR-T cells or combinatorially administered to enhance the therapeutic potential in clinical trials. In this review, we
summarize the strategies exploited by ILs to improve the anti-cancer ability of CAR-T cells and the different impacts of different ILs on CAR-T cells. Access through your institution Buy or
subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 6 print issues and online
access $259.00 per year only $43.17 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which
are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
IL-24 IMPROVES EFFICACY OF CAR-T CELL THERAPY BY TARGETING STEMNESS OF TUMOR CELLS Article 12 February 2024 CAR-T CELL THERAPY: CURRENT LIMITATIONS AND POTENTIAL STRATEGIES Article Open
access 06 April 2021 EMERGING ROLES OF CAR-NK CELL THERAPIES IN TUMOR IMMUNOTHERAPY: CURRENT STATUS AND FUTURE DIRECTIONS Article Open access 10 July 2024 REFERENCES * June C, Rosenberg SA,
Sadelain M, Weber JS. T-cell therapy at the threshold. _Nat Biotechnol_ 2012; 30: 611–614. Article CAS PubMed PubMed Central Google Scholar * Eshhar Z. The T-body approach: redirecting
T cells with antibody specificity. _Handb Exp Pharmacol_ 2008; (181: 329–342. Article CAS Google Scholar * Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA et al. Adoptive
immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. _Blood_ 2008; 112: 2261–2271. Article CAS PubMed
PubMed Central Google Scholar * Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA et al. A phase I study on adoptive immunotherapy using gene-modified T cells for
ovarian cancer. _Clin Cancer Res_ 2006; 12 (20 Pt 1): 6106–6115. Article CAS PubMed PubMed Central Google Scholar * Chmielewski M, Hombach AA, Abken H. CD28 cosignalling does not affect
the activation threshold in a chimeric antigen receptor-redirected T-cell attack. _Gene Ther_ 2011; 18: 62–72. Article CAS PubMed Google Scholar * Imai C, Mihara K, Andreansky M,
Nicholson IC, Pui CH, Geiger TL et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. _Leukemia_ 2004; 18: 676–684.
Article CAS PubMed Google Scholar * Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G et al. CD28 costimulation improves expansion and persistence of chimeric antigen
receptor-modified T cells in lymphoma patients. _J Clin Invest_ 2011; 121: 1822–1826. Article CAS PubMed PubMed Central Google Scholar * Milone MC, Fish JD, Carpenito C, Carroll RG,
Binder GK, Teachey D et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy _in vivo_. _Mol Ther_
2009; 17: 1453–1464. Article CAS PubMed PubMed Central Google Scholar * Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR et al. Chimeric antigen receptor-modified T cells
for acute lymphoid leukemia. _N Engl J Med_ 2013; 368: 1509–1518. Article CAS PubMed PubMed Central Google Scholar * Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG et
al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. _Sci Transl Med_ 2013; 5: 177ra38. Article PubMed PubMed
Central CAS Google Scholar * Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. _Blood_ 2006; 107: 2409–2414. Article
CAS PubMed PubMed Central Google Scholar * Mor F, Cohen IR. IL-2 rescues antigen-specific T cells from radiation or dexamethasone-induced apoptosis. Correlation with induction of Bcl-2.
_J Immunol_ 1996; 156: 515–522. CAS PubMed Google Scholar * Mueller DL, Seiffert S, Fang W, Behrens TW. Differential regulation of bcl-2 and bcl-x by CD3, CD28, and the IL-2 receptor in
cloned CD4+ helper T cells. A model for the long-term survival of memory cells. _J Immunol_ 1996; 156: 1764–1771. CAS PubMed Google Scholar * Rosenberg SA. IL-2: the first effective
immunotherapy for human cancer. _J Immunol_ 2014; 192: 5451–5458. Article CAS PubMed Google Scholar * Chang AE, Li Q, Jiang G, Sayre DM, Braun TM, Redman BG. Phase II trial of autologous
tumor vaccination, anti-CD3-activated vaccine-primed lymphocytes, and interleukin-2 in stage IV renal cell cancer. _J Clin Oncol_ 2003; 21: 884–890. Article CAS PubMed Google Scholar *
Li Q, Grover AC, Donald EJ, Carr A, Yu J, Whitfield J et al. Simultaneous targeting of CD3 on T cells and CD40 on B or dendritic cells augments the antitumor reactivity of tumor-primed lymph
node cells. _J Immunol_ 2005; 175: 1424–1432. Article CAS PubMed Google Scholar * Macgregor JN, Li Q, Chang AE, Braun TM, Hughes DP, McDonagh KT. _Ex vivo_ culture with interleukin
(IL)-12 improves CD8(+) T-cell adoptive immunotherapy for murine leukemia independent of IL-18 or IFN-gamma but requires perforin. _Cancer Res_ 2006; 66: 4913–4921. Article CAS PubMed
Google Scholar * Mule JJ, Shu S, Schwarz SL, Rosenberg SA. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. _Science_ 1984; 225:
1487–1489. Article CAS PubMed Google Scholar * Yannelli JR, Hyatt C, McConnell S, Hines K, Jacknin L, Parker L et al. Growth of tumor-infiltrating lymphocytes from human solid cancers:
summary of a 5-year experience. _Int J Cancer_ 1996; 65: 413–421. Article CAS PubMed Google Scholar * Emtage PC, Lo AS, Gomes EM, Liu DL, Gonzalo-Daganzo RM, Junghans RP.
Second-generation anti-carcinoembryonic antigen designer T cells resist activation-induced cell death, proliferate on tumor contact, secrete cytokines, and exhibit superior antitumor
activity _in vivo_: a preclinical evaluation. _Clin Cancer Res_ 2008; 14: 8112–8122. Article CAS PubMed PubMed Central Google Scholar * Brocker T. Chimeric Fv-zeta or Fv-epsilon
receptors are not sufficient to induce activation or cytokine production in peripheral T cells. _Blood_ 2000; 96: 1999–2001. Article CAS PubMed Google Scholar * Pinthus JH, Waks T,
Kaufman-Francis K, Schindler DG, Harmelin A, Kanety H et al. Immuno-gene therapy of established prostate tumors using chimeric receptor-redirected human lymphocytes. _Cancer Res_ 2003; 63:
2470–2476. CAS PubMed Google Scholar * Lo AS, Ma Q, Liu DL, Junghans RP. Anti-GD3 chimeric sFv-CD28/T-cell receptor zeta designer T cells for treatment of metastatic melanoma and other
neuroectodermal tumors. _Clin Cancer Res_ 2010; 16: 2769–2780. Article CAS PubMed Google Scholar * Moeller M, Haynes NM, Kershaw MH, Jackson JT, Teng MW, Street SE et al. Adoptive
transfer of gene-engineered CD4+ helper T cells induces potent primary and secondary tumor rejection. _Blood_ 2005; 106: 2995–3003. Article CAS PubMed Google Scholar * Moeller M, Kershaw
MH, Cameron R, Westwood JA, Trapani JA, Smyth MJ et al. Sustained antigen-specific antitumor recall response mediated by gene-modified CD4+ T helper-1 and CD8+ T cells. _Cancer Res_ 2007;
67: 11428–11437. Article CAS PubMed Google Scholar * Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR et al. Antitransgene rejection responses contribute to attenuated
persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. _Biol Blood Marrow Transplant_ 2010; 16: 1245–1256. Article CAS PubMed
PubMed Central Google Scholar * Junghans RP, Ma Q, Rathore R, Gomes EM, Bais AJ, Lo AS et al. Phase I trial of anti-PSMA designer CAR-T cells in prostate cancer: Possible role for
interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. _Prostate_ 2016; 76: 1257–1270. Article CAS PubMed Google Scholar * Kershaw MH, Westwood JA,
Parker LL, Wang G, Eshhar Z, Mavroukakis SA et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. _Clin Cancer Res_ 2006; 12 (20 Pt 1): 6106–6115.
Article CAS PubMed PubMed Central Google Scholar * Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V et al. Cloning of a T cell growth factor that interacts with the
beta chain of the interleukin-2 receptor. _Science_ 1994; 264: 965–968. Article CAS PubMed Google Scholar * Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R et al.
Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. _J Exp Med_ 1994; 180: 1395–1403. Article CAS PubMed Google Scholar
* Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. _Nat Rev Immunol_ 2006; 6: 595–601. Article CAS PubMed Google Scholar
* Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. _Adv Immunol_ 2005; 86: 209–239. Article CAS PubMed Google Scholar * Yamasaki
S, Maeda M, Ohshima K, Kikuchi M, Otsuka T, Harada M. Growth and apoptosis of human natural killer cell neoplasms: role of interleukin-2/15 signaling. _Leuk Res_ 2004; 28: 1023–1031. Article
CAS PubMed Google Scholar * Zhang J, Sun R, Wei H, Zhang J, Tian Z. Characterization of interleukin-15 gene-modified human natural killer cells: implications for adoptive cellular
immunotherapy. _Haematologica_ 2004; 89: 338–347. CAS PubMed Google Scholar * Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L et al. IL-2-induced
activation-induced cell death is inhibited in IL-15 transgenic mice. _Proc Natl Acad Sci USA_ 2000; 97: 11445–11450. Article CAS PubMed PubMed Central Google Scholar * Jakobisiak M,
Golab J, Lasek W. Interleukin 15 as a promising candidate for tumor immunotherapy. _Cytokine Growth Factor Rev_ 2011; 22: 99–108. Article CAS PubMed Google Scholar * Brentjens RJ,
Latouche JB, Santos E, Marti F, Gong MC, Lyddane C et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. _Nat
Med_ 2003; 9: 279–286. Article CAS PubMed Google Scholar * Numbenjapon T, Serrano LM, Singh H, Kowolik CM, Olivares S, Gonzalez N et al. Characterization of an artificial
antigen-presenting cell to propagate cytolytic CD19-specific T cells. _Leukemia_ 2006; 20: 1889–1892. Article CAS PubMed Google Scholar * Numbenjapon T, Serrano LM, Chang WC, Forman SJ,
Jensen MC, Cooper LJ. Antigen-independent and antigen-dependent methods to numerically expand CD19-specific CD8+ T cells. _Exp Hematol_ 2007; 35: 1083–1090. Article CAS PubMed Google
Scholar * Ramanayake S, Bilmon I, Bishop D, Dubosq MC, Blyth E, Clancy L et al. Low-cost generation of Good Manufacturing Practice-grade CD19-specific chimeric antigen receptor-expressing T
cells using piggyBac gene transfer and patient-derived materials. _Cytotherapy_ 2015; 17: 1251–1267. Article CAS PubMed Google Scholar * Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM,
Quigley MF et al. A human memory T cell subset with stem cell-like properties. _Nat Med_ 2011; 17: 1290–1297. Article CAS PubMed PubMed Central Google Scholar * Gattinoni L, Zhong XS,
Palmer DC, Ji Y, Hinrichs CS, Yu Z et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. _Nat Med_ 2009; 15: 808–813. Article CAS PubMed
PubMed Central Google Scholar * Xu Y, Zhang M, Ramos CA, Durett A, Liu E, Dakhova O et al. Closely related T-memory stem cells correlate with _in vivo_ expansion of CAR.CD19-T cells and
are preserved by IL-7 and IL-15. _Blood_ 2014; 123: 3750–3759. Article CAS PubMed PubMed Central Google Scholar * Nishio N, Dotti G. Oncolytic virus expressing RANTES and IL-15 enhances
function of CAR-modified T cells in solid tumors. _Oncoimmunology_ 2015; 4: e988098. Article PubMed PubMed Central CAS Google Scholar * Till BG, Jensen MC, Wang J, Chen EY, Wood BL,
Greisman HA et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. _Blood_ 2008; 112:
2261–2271. Article CAS PubMed PubMed Central Google Scholar * Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G et al. Virus-specific T cells engineered to coexpress
tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. _Nat Med_ 2008; 14: 1264–1270. Article CAS PubMed PubMed Central Google Scholar *
Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. _Curr Opin Immunol_ 2009; 21: 215–223. Article CAS PubMed PubMed Central Google
Scholar * Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B
lymphocyte-derived malignant cells. _Blood_ 2006; 108: 3890–3897. Article CAS PubMed PubMed Central Google Scholar * Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N
et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances _in vivo_ persistence and antitumor efficacy of adoptively transferred T cells. _Cancer Res_
2006; 66: 10995–11004. Article CAS PubMed Google Scholar * Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single
chimeric TCRzeta /CD28 receptor. _Nat Biotechnol_ 2002; 20: 70–75. Article CAS PubMed Google Scholar * Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL et al. Chimeric
receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. _Leukemia_ 2004; 18: 676–684. Article CAS PubMed Google Scholar * Milone MC,
Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic
efficacy _in vivo_. _Mol Ther_ 2009; 17: 1453–1464. Article CAS PubMed PubMed Central Google Scholar * Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM et al. Control
of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. _Proc Natl Acad Sci USA_ 2009; 106: 3360–3365. Article CAS PubMed
PubMed Central Google Scholar * Tammana S, Huang X, Wong M, Milone MC, Ma L, Levine BL et al. 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells
against B-cell malignancies. _Hum Gene Ther_ 2010; 21: 75–86. Article CAS PubMed PubMed Central Google Scholar * Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X et al. A
herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. _J Immunol_ 2009; 183: 5563–5574.
Article CAS PubMed Google Scholar * Wang J, Jensen M, Lin Y, Sui X, Chen E, Lindgren CG et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell
receptor possessing CD28 and CD137 costimulatory domains. _Hum Gene Ther_ 2007; 18: 712–725. Article CAS PubMed Google Scholar * Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM,
Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. _Mol Ther_ 2005; 12: 933–941. Article CAS PubMed
Google Scholar * Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their
anti-lymphoma/leukemia effects and safety. _Leukemia_ 2010; 24: 1160–1170. Article CAS PubMed PubMed Central Google Scholar * Huntington ND, Puthalakath H, Gunn P, Naik E, Michalak EM,
Smyth MJ et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. _Nat Immunol_ 2007; 8: 856–863. Article CAS PubMed PubMed
Central Google Scholar * Bianchi T, Gasser S, Trumpp A, MacDonald HR. c-Myc acts downstream of IL-15 in the regulation of memory CD8 T-cell homeostasis. _Blood_ 2006; 107: 3992–3999.
Article CAS PubMed Google Scholar * Wu TS, Lee JM, Lai YG, Hsu JC, Tsai CY, Lee YH et al. Reduced expression of Bcl-2 in CD8+ T cells deficient in the IL-15 receptor alpha-chain. _J
Immunol_ 2002; 168: 705–712. Article CAS PubMed Google Scholar * Wang W, Ma Y, Li J, Shi HS, Wang LQ, Guo FC et al. Specificity redirection by CAR with human VEGFR-1 affinity endows T
lymphocytes with tumor-killing ability and anti-angiogenic potency. _Gene Ther_ 2013; 20: 970–978. Article CAS PubMed Google Scholar * Berger C, Berger M, Hackman RC, Gough M, Elliott C,
Jensen MC et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. _Blood_ 2009; 114: 2417–2426. Article CAS PubMed PubMed Central Google Scholar * Munger W,
DeJoy SQ, Jeyaseelan R, Sr., Torley LW, Grabstein KH, Eisenmann J et al. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: comparison
with interleukin-2. _Cell Immunol_ 1995; 165: 289–293. Article CAS PubMed Google Scholar * Wilkie S, Burbridge SE, Chiapero-Stanke L, Pereira AC, Cleary S, van der Stegen SJ et al.
Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. _J Biol Chem_ 2010; 285: 25538–25544. Article CAS PubMed PubMed
Central Google Scholar * Papa S, van Schalkwyk M, Maher J. Clinical evaluation of ErbB-targeted CAR T-cells, following intracavity delivery in patients with ErbB-expressing solid tumors.
_Methods Mol Biol_ 2015; 1317: 365–382. Article PubMed Google Scholar * Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. _Curr Opin Immunol_ 2007; 19: 320–326.
Article CAS PubMed Google Scholar * Gargett T, Brown MP. Different cytokine and stimulation conditions influence the expansion and immune phenotype of third-generation chimeric antigen
receptor T cells specific for tumor antigen GD2. _Cytotherapy_ 2015; 17: 487–495. Article CAS PubMed Google Scholar * Casucci M, Nicolis di Robilant B, Falcone L, Camisa B, Norelli M,
Genovese P et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. _Blood_ 2013; 122: 3461–3472. Article CAS PubMed Google
Scholar * Markley JC, Sadelain M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. _Blood_ 2010; 115:
3508–3519. Article CAS PubMed PubMed Central Google Scholar * Sun JC, Lehar SM, Bevan MJ. Augmented IL-7 signaling during viral infection drives greater expansion of effector T cells
but does not enhance memory. _J Immunol_ 2006; 177: 4458–4463. Article CAS PubMed Google Scholar * Perna SK, Pagliara D, Mahendravada A, Liu H, Brenner MK, Savoldo B et al. Interleukin-7
mediates selective expansion of tumor-redirected cytotoxic T lymphocytes (CTLs) without enhancement of regulatory T-cell inhibition. _Clin Cancer Res_ 2014; 20: 131–139. Article CAS
PubMed Google Scholar * Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. _Immunity_ 2003; 19: 641–644.
Article CAS PubMed Google Scholar * Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. _Nat Rev Immunol_ 2003; 3: 133–146. Article CAS PubMed
Google Scholar * Trinchieri G. Immunobiology of interleukin-12. _Immunol Res_ 1998; 17: 269–278. Article CAS PubMed Google Scholar * Hsieh CS, Macatonia SE, Tripp CS, Wolf SF,
O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. _Science_ 1993; 260: 547–549. Article CAS PubMed Google Scholar *
Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. _J Exp Med_
2003; 197: 1141–1151. Article CAS PubMed PubMed Central Google Scholar * Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic
cells: the concept of a third signal. _Immunol Today_ 1999; 20: 561–567. Article CAS PubMed Google Scholar * Yoo JK, Cho JH, Lee SW, Sung YC. IL-12 provides proliferation and survival
signals to murine CD4+ T cells through phosphatidylinositol 3-kinase/Akt signaling pathway. _J Immunol_ 2002; 169: 3637–3643. Article CAS PubMed Google Scholar * Hendrzak JA, Brunda MJ.
Antitumor and antimetastatic activity of interleukin-12. _Curr Topics Microbiol Immunol_ 1996; 213 (Pt 3): 65–83. CAS Google Scholar * Wigginton JM, Gruys E, Geiselhart L, Subleski J,
Komschlies KL, Park JW et al. IFN-gamma and Fas/FasL are required for the antitumor and antiangiogenic effects of IL-12/pulse IL-2 therapy. _J Clin Invest_ 2001; 108: 51–62. Article CAS
PubMed PubMed Central Google Scholar * Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB et al. Effects of single-dose interleukin-12 exposure on
interleukin-12-associated toxicity and interferon-gamma production. _Blood_ 1997; 90: 2541–2548. CAS PubMed Google Scholar * Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of
interleukin-12: a review. _Toxicol Pathol_ 1999; 27: 58–63. Article CAS PubMed Google Scholar * Kang WK, Park C, Yoon HL, Kim WS, Yoon SS, Lee MH et al. Interleukin 12 gene therapy of
cancer by peritumoral injection of transduced autologous fibroblasts: outcome of a phase I study. _Hum Gene Ther_ 2001; 12: 671–684. Article CAS PubMed Google Scholar * Heinzerling L,
Burg G, Dummer R, Maier T, Oberholzer PA, Schultz J et al. Intratumoral injection of DNA encoding human interleukin 12 into patients with metastatic melanoma: clinical efficacy. _Hum Gene
Ther_ 2005; 16: 35–48. Article CAS PubMed Google Scholar * Chmielewski M, Hombach AA, Abken H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible
cytokine to modulate the tumor stroma. _Immunol Rev_ 2014; 257: 83–90. Article CAS PubMed Google Scholar * Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full
activation of naive CD8 T cells: dissociating proliferation and development of effector function. _J Exp Med_ 2003; 197: 1141–1151. Article CAS PubMed PubMed Central Google Scholar *
Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. _Mol Ther_ 2011; 19:
751–759. Article CAS PubMed PubMed Central Google Scholar * Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells
eradicate ovarian tumors. _Oncoimmunology_ 2015; 4: e994446. Article PubMed PubMed Central CAS Google Scholar * Chinnasamy D, Yu Z, Kerkar SP, Zhang L, Morgan RA, Restifo NP et al.
Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. _Clin Cancer Res_ 2012; 18: 1672–1683. Article CAS PubMed PubMed
Central Google Scholar * Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an
antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. _Cancer Res_ 2011; 71: 5697–5706. Article CAS PubMed Google Scholar * You F, Jiang L,
Zhang B, Lu Q, Zhou Q, Liao X et al. Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified Anti-MUC1 chimeric
antigen receptor transduced T cells. _Sci China Life Sci_ 2016; 59: 386–397. Article CAS PubMed Google Scholar * Koneru M, O'Cearbhaill R, Pendharkar S, Spriggs DR, Brentjens RJ. A
phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. _J Trans Med_ 2015; 13: 102. Article
CAS Google Scholar * Fan H, Walters CS, Dunston GM, Tackey R. IL-12 plays a significant role in the apoptosis of human T cells in the absence of antigenic stimulation. _Cytokine_ 2002; 19:
126–137. Article CAS PubMed Google Scholar * Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic
tumors without need for prior conditioning. _Blood_ 2012; 119: 4133–4141. Article CAS PubMed PubMed Central Google Scholar * Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu
Z et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. _Cancer Res_ 2010; 70: 6725–6734. Article CAS PubMed PubMed Central
Google Scholar * Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. _Nat Rev Drug Discov_ 2014; 13: 379–395. Article CAS PubMed Google Scholar *
Hashmi MH, Van Veldhuizen PJ. Interleukin-21: updated review of Phase I and II clinical trials in metastatic renal cell carcinoma, metastatic melanoma and relapsed/refractory indolent
non-Hodgkin's lymphoma. _Expert Opin Biol Ther_ 2010; 10: 807–817. Article CAS PubMed Google Scholar * Li Y, Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of
antigen-specific CD8+ cytotoxic T lymphocytes. _Blood_ 2008; 111: 229–235. Article PubMed PubMed Central CAS Google Scholar * Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA.
IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. _J Immunol_ 2004; 173: 900–909. Article CAS PubMed
Google Scholar * Singh H, Figliola MJ, Dawson MJ, Olivares S, Zhang L, Yang G et al. Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using
Sleeping Beauty system and artificial antigen presenting cells. _PLoS ONE_ 2013; 8: e64138. Article CAS PubMed PubMed Central Google Scholar * Singh H, Figliola MJ, Dawson MJ, Huls H,
Olivares S, Switzer K et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. _Cancer Res_ 2011; 71: 3516–3527. Article
CAS PubMed PubMed Central Google Scholar * Deniger DC, Yu J, Huls MH, Figliola MJ, Mi T, Maiti SN et al. Sleeping beauty transposition of chimeric antigen receptors targeting receptor
tyrosine kinase-like orphan receptor-1 (ROR1) into diverse memory T-cell populations. _PLoS ONE_ 2015; 10: e0128151. Article PubMed PubMed Central CAS Google Scholar * Cherkassky L,
Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. _J Clin Invest_ 2016; 126:
3130–3144. Article PubMed PubMed Central Google Scholar Download references This work was supported by the National High-tech R&D program (863 Program) 2014AA020704 and the National
Natural and Scientific Foundation of China, 81572981/H1611, 81400057/H0111, 81123003/H1604 and 81201789/H1611. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * State Key Laboratory of
Biotherapy/Collaborative Center for Biotherapy, West China Hospital, Sichuan University, Chengdu, China Y Huang, D Li, D-Y Qin, Y-Q Wei & W Wang * Department of Medical Oncology, Cancer
Center, State Key Laboratory of Biotherapy, West China Hospital, West China Medical School, Sichuan University, Chengdu, China H-F Gou * Department of Emergency, West China Hospital, Sichuan
University, Chengdu, China W Wei * Department of Thoracic Oncology, Cancer Center and State Key Laboratory of Biotherapy, West China Hospital, West China Medical School, Sichuan University,
Chengdu, China Y-S Wang Authors * Y Huang View author publications You can also search for this author inPubMed Google Scholar * D Li View author publications You can also search for this
author inPubMed Google Scholar * D-Y Qin View author publications You can also search for this author inPubMed Google Scholar * H-F Gou View author publications You can also search for this
author inPubMed Google Scholar * W Wei View author publications You can also search for this author inPubMed Google Scholar * Y-S Wang View author publications You can also search for this
author inPubMed Google Scholar * Y-Q Wei View author publications You can also search for this author inPubMed Google Scholar * W Wang View author publications You can also search for this
author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to W Wang. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare no conflict of interest. RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Huang, Y., Li, D., Qin, DY. _et al._ Interleukin-armed chimeric antigen receptor-modified T cells for cancer immunotherapy.
_Gene Ther_ 25, 192–197 (2018). https://doi.org/10.1038/gt.2017.81 Download citation * Received: 01 January 2017 * Revised: 10 April 2017 * Accepted: 28 July 2017 * Published: 21 September
2017 * Issue Date: June 2018 * DOI: https://doi.org/10.1038/gt.2017.81 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
