Antifungal effect of gatifloxacin and copper ions combination
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT It is a well-known fact that to bring a new molecule it may take more than a decade. The existing drugs, which are known for their adverse reaction or toxicity, if utilized and
allowed in different formulation, the new effective formulation may be discovered and developed. This may help in reducing various side effects, time and costs. In this study, fungal
infection was inoculated superficially over the skin of guinea pigs and treated with the broad-spectrum antimicrobial (gatifloxacin) in combination with non-toxic and effective amount of
copper ions. MIC of copper (0.20%) was also determined. Concentration of gatifloxacin (100 μg ml−1) with the combination of copper ions (MIC) at which it inhibits the visible growth of
fungal strains was also evaluated. Hematological parameters, such as total leukocyte count and differential leukocyte count, were evaluated. The results have shown increase in these
parameters after fungal infection, which reaches its normal value after treatment with the combination of gatifloxacin and copper ions. Outcomes of the research concluded that gatifloxacin
100 μg ml−1 can be used by 0.20% of copper ions to prevent growth of some fungal strains (_Candida albicans_ and _Aspergillus niger_), which causes skin infections with more potency. SIMILAR
CONTENT BEING VIEWED BY OTHERS THE STUDY OF TRYPTOPHOL CONTAINING EMULGEL ON FUNGAL REDUCTION AND SKIN IRRITATION Article Open access 02 November 2023 ANTIMICROBIAL AND CYTOTOXIC ACTIVITY
OF GREEN SYNTHESIS SILVER NANOPARTICLES TARGETING SKIN AND SOFT TISSUE INFECTIOUS AGENTS Article Open access 15 July 2021 ANTIBACTERIAL ACTIVITY AND PHYTOCHEMICAL COMPONENTS OF LEAF EXTRACT
OF _CALPURNIA AUREA_ Article Open access 16 June 2023 INTRODUCTION Superficial fungal infections are common worldwide. These infections occur in both healthy and immunocompromised
patients.1, 2 They are believed to affect 20–25% of the world’s population, and the incidence continues to increase. They are predominantly caused by dermatophytes, yeasts and moulds and the
causative species vary by geographic region. The prevalence of fungal infections has been suggested for the increase with age and to be present at a rate of about 5% in people aged 55 years
and older.3, 4 However, the increasing administration of antifungal agents to treat fungal infections has led to the development of fungal resistance. The emergence of resistance shows the
necessity of discovering new antifungal agents with broader antifungal spectra, low toxicity and higher therapeutic indexes.5, 6 Copper is an essential trace element for normal plant growth
and development. However, an excessive amount of copper in soil is highly toxic to both higher plants and microorganisms.7, 8 If these ions are combined with the existing antimicrobial
agents in limited quantity, then the combination can be used as an antifungal drug. Monotherapy is the usual treatment for invasive fungal infections, owing to lack of safe, effective
combination of antifungal drugs. However, the benefits of a well-tolerated, synergistic combination would be great—the enhanced efficacy would improve clinical outcome, reduce the need for
prolonged courses of treatment and prevent the emergence of antifungal drug resistance. Combination therapy is therefore being suggested as a means of combating resistance and improving
clinical outcome, just as it is for serious bacterial infections. Antifungal antibodies would be a natural partner in a combinatorial approach to antifungal therapy.9, 10, 11 DNA gyrase is a
member of the group of enzymes known as DNA topoisomerases that catalyze changes in DNA topology.12 Gyrase is the only member of this group that can introduce negative supercoils into DNA
at the expense of ATP hydrolysis.13 DNA gyrase is essential in bacteria and is the target of a number of antimicrobial agents.14 Gatifloxacin is a broad-spectrum anti-infective agent of the
fluoroquinolone class. It is most active against aerobic Gram-negative organisms including enteric pathogens. Gatifloxacin is bactericidal via inhibition of DNA gyrase, an enzyme responsible
for counteracting the excessive supercoiling of DNA during replication or transcription.15 Cu2+ ions are transported by the uptake system for essential metal ions to the cell where they can
accumulate and exert toxic effects at high concentrations.16 Copper’s initial site of action is considered to be at the plasma membrane. It has been shown that exposure of fungi and yeast
to elevated copper concentrations can lead to a rapid decline in membrane integrity. This generally manifests itself as leakage of mobile cellular solutes (for example, K+) and cell
death.17, 18 Similar effects reported in higher organisms have now been largely attributed to the redox-active nature of copper and the ability of copper to catalyze the generation of free
radicals and promote membrane lipid peroxidation.19 Copper may damage many proteins. This may occur via displacement of essential metals from their native binding sites in the proteins, or
via direct interactions with the proteins. Copper also may mediate free radical attack of amino acids, especially of histidine and proline, causing substantial protein alterations and even
protein cleavage.20, 21, 22 Copper ion has a specific affinity for DNA and can bind with helical structure of DNA, leading to cross-linking the strands. Copper reversibly denatures DNA in
low ionic strength solutions competing with the hydrogen bonding present within the DNA molecule. Thus, copper binding to DNA implies that nucleic acid degradation by generating several OH–
radicals near the binding site causing multiple damage to the nucleic acids.23, 24 Our hypothesis was based on the fact that gatifloxacin inhibits DNA gyrase in bacteria and it cannot
penetrate the fungal membrane because of the presence of ergosterol on fungal membrane. Copper ions penetrate the membrane; the pore may be created on fungal membrane. In this study, copper
ions are combined with gatifloxacin in a very small amount. These copper ions will create pores on the fungal membrane by disrupting the ergosterol present on the membrane and hence will
help gatifloxacin in penetrating the fungal membrane where it will bind with DNA topoisomerases that are present inside the fungus and will inhibit it. This combination can be used in the
treatment of fungal infection. MATERIALS AND METHODS ANIMALS Four healthy male guinea pigs weighing approximately 500 g were obtained from the animal house facility at Siddhartha Institute
of Pharmacy, Dehradun, India. They were housed in individual ventilated cages under the conditions of 22–25 °C and a 12-h light–dark cycle, with free access to food and water. The
experimental protocol was approved by the Institutional Animal Ethical Committee (IAEC approval number-SIP/IAEC/2/A/2011) as per the guidance of Committee for the purpose of Control and
Supervision of Experiments on Animals, Ministry of Social Justice and Empowerment, Government of India. CHEMICALS AND DRUGS Gatifloxacin was procured from Cipla, Sikkim, India, as a gift
sample for study. Fungal strains (_Aspergillus niger_ and _Candida albicans_) were obtained from Sardar Bhagwan Singh College of Pharmacy with strain no. MTCC (282,183), Dehradun, India. _IN
VITRO_ STUDY DETERMINATION OF MIC OF COPPER MIC was determined by incorporating various concentrations of copper (0.05, 0.10, 0.15 and 0.20%) in Sabouraud Dextrose broth. In all, 100 μl of
fungal inoculum was added to each tube and incubated at room temperature for 7 days. The MIC is regarded as the lowest concentration that does not permit any visible growth after 7–21 days
of inoculation. DETERMINATION OF ANTIFUNGAL SUSCEPTIBILITY OF GATIFLOXACIN Culture media for fungus were prepared by dissolving 6.5 g of Sabouraud Dextrose Agar in a conical flask by adding
50 ml of distilled water, autoclaved at 15 lbs (121 °C) for 15 min and cooled. The MIC of copper obtained was added in each flask. Sterilized media were transferred aseptically (by using
laminar air flow) into the sterilized glass Petri dishes. When the media were solidified, Petri dishes were cultured with different strains of fungus (_Candida albicans_ and _Aspergillus
niger_). Different concentration of gatifloxacin (20, 50 and 100 μg ml−1) was added on half the portion of each Petri plate and kept in the incubator at 28 °C observed after 1st, 3rd, 5th,
7th, 9th and 10th day. DETERMINATION OF ANTIFUNGAL SUSCEPTIBILITY OF COMBINATION Combination of 25 μg ml−1 gatifloxacin with 0.20%, 50 μg ml−1 gatifloxacin +0.20% and 100 μg ml−1
gatifloxacin +0.20% copper was used to determine fungal growth inhibition on culture media. In all, 100 μg ml−1 gatifloxacin +0.20% copper was effective in inhibiting fungal growth
effectively. Hence, this dose combination was selected for further study on guinea pigs. _IN VIVO_ STUDY ON GUINEA PIG INDUCTION OF FUNGAL INFECTION Animals were divided into two groups,
each having two animals. The first group was normal control. Second was treatment control group. In the second group, fungal spores were inoculated superficially and treatment was done with
the combination of copper ions and gatifloxacin. The lesions were clinically followed-up daily until resolution was observed. After the 15th day of infection, animals were treated. Animals
were inoculated according to the modified method of Shimamura _et al._25 The hair in the posterior dorsal region of the animals was removed with a sterile scalpel blade. Fungal strains were
suspended in sterile water and were inoculated at the site, which was covered with a polyethylene film and kept in place with a high elastic bandage for 24 h. After 24 h, the bandage was
removed, the area was cleared and sterilized with ethyl alcohol and water and the animals were cased individually for 15 days with adequate supply of food and water. TREATMENT Treatment was
done with microemulsion. For preparing the microemulsion, 40% oil, 10% surfactant (Tween80) and 50% water were mixed together with the concentration of gatifloxacin at which it inhibits the
visible growth of fungus (100 μg ml−1) and the MIC of copper (0.20%), homogenized with the help of homogenizer and are stored in glass vials until use. Induction of infection was confirmed
on the 15th day of inoculation. The treatment of the animals was started after confirmation of the infection. The infected areas were applied with microemulsion twice daily in the morning
and afternoon. Treatment continued regularly at the same time until complete recovery was achieved. HEMATOLOGICAL EVALUATION Whole blood was collected from the animals into EDTA bottles and
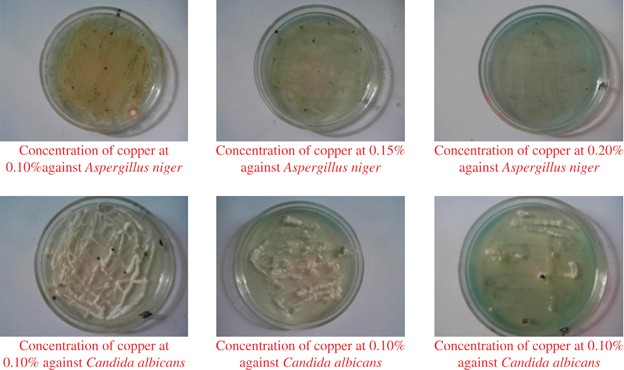
assayed for the total leukocyte count (TLC) and differential leukocyte count (DLC) using standard laboratory technique. RESULTS MIC OF COPPER _Aspergillus niger_ and _Candida albicans_ were
able to tolerate copper metal ion in the growth medium up to 0.10% and 0.15%, respectively, but inhibited to grow at 0.20%. Fungal growth was increased at 0.10% (Table 1 and Figure 1) but
then significantly decreased with increased copper concentrations in the medium (Tables 2 and 3 and Figure 1). The data shown in the tables below depict that copper ions in the concentration
of 0.10% and 0.15%, respectively, were not effective for _Aspergillus niger_ and _Candida albicans_. The data depict that copper ions in the concentration of 0.20% were sufficient to
prevent the growth of both fungal strains. Hence, we considered it as the MIC of copper ion for preventing the growth of all strains of fungus. _IN VITRO_ ANTIFUNGAL SUSCEPTIBILITY TEST OF
COMBINATION OF DRUG As shown in Tables 4 and 5 and Figure 2, combination of 25 μg ml−1 gatifloxacin with 0.20% copper and combination of 50 μg ml−1 gatifloxacin +0.20% copper were not
effective to inhibit the growth of fungus, but the data shown in the Table 6 shows that combination of 100 μg ml−1 gatifloxacin +0.20% copper was sufficient to inhibit the strains of fungus
under observation. Hence, from the above data, we consider that 100 μg ml−1 gatifloxacin can be combined with 0.20% copper ions to prevent growth of fungal strains. GUINEA PIG MODEL OF
FUNGAL INFECTION Experimental infection of guinea pigs with _Aspergillus niger_ and _Candida albicans_ resulted in lesions in animals. The first signs of infection were observed on the 5th
day after inoculation, which are manifested in the form of edema, erythema and mild shedding. These alterations became more evident around the 10th day. The lesions progressively increased
and were found to be covered with white-yellow crusts strongly adhered to the epidermis between the 8th and 15th day. Treatment procedure was started after the 15th day. Microemulsion was
applied topically over the fungal-infected site of the guinea pig twice at morning and afternoon. Lesions were formed where the skin was abraded and did not extend to other sites of the
body. Treatment causes little removal of crusts, erythema and little gain in hair on the 8th day of treatment. The effects were more marked on day 10 with clearer skin and more hair. On day
14, the treatment showed complete removal of infected material (crusts, large skin flakes, shedding and erythema) with hair growth. After the treatment, skin looked clearer, moisturized,
soft and elastic. TOTAL LEUKOCYTE COUNT The TLC in fungal-infected guinea pigs was increased as compared with the normal group. After treatment with the combination of 100 μg ml−1
gatifloxacin and 0.20% copper ion, TLC of guinea pigs reached to its normal value (Table 7 and Figure 3). DIFFERENTIAL LEUKOCYTE COUNT Eosinophils, monocytes, neutrophils and lymphocytes
showed an increase in fungal-infected guinea pigs. After treatment with a combination of 100 μg ml−1 gatifloxacin and 0.20% copper ion, eosinophils, monocytes, neutrophils and lymphocytes
reached to their normal level (Table 8 and Figure 4). DISCUSSION AND CONCLUSION Summarizing the results, we can say that the present work on fungal infection in guinea pigs represents a
relatively simple model to perform the study of the efficacy of the combination of gatifloxacin and copper ions as an antifungal agent. Over the past decade, superficial mycoses of the
glabrous skin are among the most prevalent of human infectious diseases seen in clinical practice.26 There are lots of weaknesses in spectrum, potency, safety and pharmacokinetic properties
of existing antifungal agents. When faced with antifungal drugs, fungal pathogens have, in principle, the capacity to overcome their inhibitory action through specific resistance
mechanisms.11 This significant clinical failure has led to the development of new antifungal agents, the tendency to increase the administered dose of antifungal agent, and to the
combination of two or more agents.10 Gatifloxacin is bactericidal via inhibition of DNA gyrase, an enzyme responsible for counteracting the excessive supercoiling of DNA during replication
or transcription.15 Copper’s initial site of action is considered to be at the plasma membrane. It has been shown that exposure of fungi and yeast to elevated copper concentrations can lead
to a rapid decline in membrane integrity. This generally manifests itself as leakage of mobile cellular solutes (for example, K+) and cell death.17, 18 From this viewpoint in this study, we
have treated the fungal-infected guinea pigs with the combination of gatifloxacin and copper ions at low concentration in the form of microemulsion because of their stability and their
considerable potential to act as drug delivery vehicles.27, 28, 29 As gatifloxacin cannot penetrate the fungal membrane because of the presence of ergosterol on the fungal membrane, which
serves as a bioregulator of membrane fluidity and asymmetry and consequently membrane integrity.30 Hence, we used copper ions for the penetration of gatifloxacin inside the fungal membrane.
The results suggest that copper ions at low concentration create micropores on the fungal membrane by disrupting the ergosterol in fungal cells by the leakage of transmembrane proteins,
essential solutes from the fungal membrane and hence leads to the disruption of membrane integrity. This assisted gatifloxacin in penetrating the fungal cell wall through the micropores.
Gatifloxacin after penetration inhibits DNA topoisomerase I and II that are present in the fungus28, 31 and hence prevents its replication. This causes the denaturation of DNA inside the
fungus. This suggests its fungicidal action. Previously, researchers reported that sensitization to _Aspergillus_ increased eosinophils and total serum immunoglobulin E levels,32 moreover
some others found in their investigations that total eosinophil counts and lymphocyte counts of infected fishes decreased significantly (43% and 36%). When the DLCs were performed, the
lymphocytes decreased in infected groups (9%, 11%) whereas neutrophils increased.33 TLC and DLC parameters are used as a specific marker during diagnosis in the early detection of fungal
infection. From this study, it can be concluded that the exposure of fungal infection to guinea pigs leads to the increase in the TLC and DLC parameters significantly (_P_⩽0.001) (Tables 7
and 8) as compared with a normal control group. The TLC and DLC levels decreased significantly (_P_⩽0.001) and reached toward the normal range in the treatment group treated combination
therapy of gatifloxacin and copper ions. Thus, from this study, this combination of copper and gatifloxacin prevents the fungal growth, which further helps in controlling the infections
(Figures 3, 4, 5). The data obtained from this study performed in a combination of gatifloxacin with copper ions to screen its antifungal activity, it is concluded that a combination of
0.20% of copper ions with 100 μg ml−1 gatifloxacin was able to treat the fungal infection and decreased the hematological markers, which were increased during fungal infection. This will
open new perspectives that this combination therapy of gatifloxacin with copper ions will prevent, slow or treat the occurrence of fungal infection. REFERENCES * Pierard, G. E., Arrese, J.
E. & Pierard, F. C. Treatment and prophylaxis of tinea infections. _Drugs_ 52, 209–224 (1996). Article CAS PubMed Google Scholar * Rudy, S. J. Superficial fungal infections in
children and adolescents. _Nurse Pract. Forum_ 10, 56–66 (1999). CAS PubMed Google Scholar * Ameen, M. Epidemiology of superficial fungal infections. _Clin. Dermatol._ 28, 197–201 (2010).
Article PubMed Google Scholar * Havlickova, B., Czaika, V. A. & Friedrich, M. Epidemiological trends in skin mycoses worldwide. _Mycoses_ 51, 2–15 (2008). Article PubMed Google
Scholar * Yu, S. _et al_. Synthesis and antifungal evaluation of novel triazole derivatives as inhibitors of cytochrome P450 14a-demethylase. _Eur. J. Med. Chem._ 45, 4435–4445 (2010).
Article CAS PubMed Google Scholar * Sanglard, D. Resistance of human fungal pathogens to antifungal drugs. _Curr. Opin. Microbiol._ 5, 379–385 (2002). Article CAS PubMed Google
Scholar * Wong, M. H. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. _Chemosphere_ 50, 775–780 (2003). Article CAS PubMed Google Scholar *
Bolan, N. S., Adriano, D., Mani, S. & Khan, A. Adsorption, complexation, and phytoavailability of copper as influenced by organic manure. _Environ. Toxicol. Chem._ 22, 450–456 (2003).
Article CAS PubMed Google Scholar * Matthews, R. C. & Burnie, J. P. Recombinant antibodies: a natural partner in combinatorial antifungal therapy. _Vaccine_ 22, 865–871 (2004).
Article CAS PubMed Google Scholar * Fohrer, C. _et al_. Antifungal combination treatment: a future perspective. _Int. J. Antimicrob. Agents_ 27S, S25–S30 (2006). Article Google Scholar
* Kontoyiannis, D. P. & Lewis, R. E. Antifungal drug resistance of pathogenic fungi. _Lancet_ 359, 1135–1144 (2002). Article CAS PubMed Google Scholar * Corbett, K. D. &
Berger, J. M. Structure, molecular mechanisms, and evolutionary relationships in DNA topoisomerases. _Annu. Rev. Biophys. Biomol. Struct._ 33, 95–118 (2004). Article CAS PubMed Google
Scholar * Parks, W. M., Bottrill, A. R., Pierrat, O. A., Durrant, M. C. & Maxwell, A. The action of the bacterial toxin, microcin B17, on DNA gyrase. _Biochimie_ 89, 500–507 (2007).
Article CAS PubMed Google Scholar * Maxwell, A. DNA gyrase as a drug target. _Trends. Microbiol._ 5, 102–109 (1997). Article CAS PubMed Google Scholar * Nawaz, H., Rauf, S., Akhtar,
K. & Khalid, A. M. Electrochemical DNA biosensor for the study of gatifloxacin–DNA interaction. _Anal. Biochem._ 354, 28–34 (2006). Article CAS PubMed Google Scholar * Malachova, K.,
Praus, P., Rybkova, Z. & Ondrej, K. Antibacterial and antifungal activities of silver, copper and zinc montmorillonites. _Appl. Clay Sci._ 53, 642–645 (2011). Article CAS Google
Scholar * Cervantes, C. & Gutierrez-Corona, F. Copper resistance mechanisms in bacteria and fungi. _FEMS Microbiol_ 14, 121–137 (1994). Article CAS Google Scholar * Ohsumi, Y.,
Kitamoto, K. & Anraku, Y. J. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. _J. Bacteriol._ 170, 2676–2682 (1988). Article CAS PubMed PubMed
Central Google Scholar * Stohs, S. J. & Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. _Free Radic. Biol. Med._ 18, 321–336 (1995). Article CAS PubMed Google Scholar
* Kim, J. H., Cho, H., Ryu, S. E. & Choi, M. U. Effects of metal ions on the activity of protein tyrosine phosphatase VHR: highly potent and reversible oxidative inactivation by Cu2+
ion. _Arch. Biochem. Biophys._ 382, 72–80 (2000). Article CAS PubMed Google Scholar * Davies, M. J., Gilbert, B. C. & Haywood, R. M. Radical-induced damage to proteins: e.s.r.
spin-trapping studies. _Free Radic. Res. Commun._ 15, 111–127 (1991). Article CAS PubMed Google Scholar * Dean, R. T., Wolff, S. P. & McElligott, M. A. Histidine and proline are
important sites of free radical damage to proteins. _Free Radic. Res. Commun._ 7, 97–103 (1989). Article CAS PubMed Google Scholar * Sagripanti, J. L., Goering, P. L. & Lamanna, A.
Interaction of copper with DNA and antagonism by other metals. _Toxicol. Appl. Pharmacol._ 110, 477–485 (1991). Article CAS PubMed Google Scholar * Sagripanti, J. L. & Kraemer, K. H.
Site-specific oxidative DNA damage at polyguanosines produced by copper plus hydrogen peroxide. _J. Biol. Chem._ 264, 1729–1734 (1989). CAS PubMed Google Scholar * Shimamura, T., Kubota,
N. & Shibuya, K. Animal model of dermatophytosis. _J. Biomed. Biotechnol._ 2012, 1–11 (2012). Article Google Scholar * Garg, J. _et al_. Rapid detection of dermatophytes from skin and
hair. _BMC Res. Notes_ 2, 1–6 (2009). Article Google Scholar * Grampurohit, N., Ravikumar, P. & Mallya, R. Microemulsions for topical use– a review. _Indian J. Pharm. Educ. Res._ 45,
100–107 (2011). Google Scholar * Wang, J. C. Cellular roles of DNA Topoisomerases. A Molecular Perspective. _Nat. Rev. Mol. Cell. Biol._ 3, 430–440 (2002). Article CAS Google Scholar *
Lawrence, M. J. & Rees, G. D. Microemulsion-based media as novel drug delivery systems. _Adv. Drug Deliv. Rev._ 45, 89–121 (2000). Article CAS PubMed Google Scholar * Howell, S. A.,
Moorea, M. K., Mallet, I. & Noble, W. C. Sterols of fungi responsible for superficial skin and nail infection. _J. Gen. Microbiol._ 136, 241–247 (1990). Article CAS PubMed Google
Scholar * Burden, D. A. & Osheroff, N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. _Biochim. Biophys. Acta_ 1400, 139–154 (1998). Article CAS
PubMed Google Scholar * Ma, Y. L. _et al_. Prevalence of allergic bronchopulmonary aspergillosis in Chinese patients with bronchial asthma. _Zhonghua. Jie. He. He. Hu. Xi. Za. Zhi._ 34,
909–913 (2011). PubMed Google Scholar * Innocent, B. X., Fathima, M. S. A. & Sivagurunathan, A. Haematology of _Cirrhinus mrigala_ fed with vitamin C supplemented diet and post
challenged by _Aphanomyces invadens_. _J. Appl. Pharm. Sci._ 1, 141–144 (2011). Google Scholar Download references AUTHOR INFORMATION Author notes * Saiba Shams: Former address: Hygia
Institute of Pharmaceutical Education and Research, Ghaila Rd, Lucknow, Uttar Pradesh, India. AUTHORS AND AFFILIATIONS * Department of Pharmacology, Siddhartha Institute of Pharmacy,
Dehradun, India Saiba Shams, Muhammad Afzal, Imran Kazmi & Firoz Anwar * Department of Pharmacognosy, College of Dental Medicines and Pharmacy, Buraydah, Saudi Arabia Babar Ali *
Department of Biochemistry, Faculty of Sciences, King Abdulaziz University, Jeddah, Saudi Arabia Fahad A Al-Abbasi Authors * Saiba Shams View author publications You can also search for this
author inPubMed Google Scholar * Babar Ali View author publications You can also search for this author inPubMed Google Scholar * Muhammad Afzal View author publications You can also search
for this author inPubMed Google Scholar * Imran Kazmi View author publications You can also search for this author inPubMed Google Scholar * Fahad A Al-Abbasi View author publications You
can also search for this author inPubMed Google Scholar * Firoz Anwar View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS
Correspondence to Muhammad Afzal, Imran Kazmi or Firoz Anwar. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Shams, S., Ali, B., Afzal, M. _et al._
Antifungal effect of Gatifloxacin and copper ions combination. _J Antibiot_ 67, 499–504 (2014). https://doi.org/10.1038/ja.2014.35 Download citation * Received: 17 July 2013 * Revised: 26
January 2014 * Accepted: 26 February 2014 * Published: 04 June 2014 * Issue Date: July 2014 * DOI: https://doi.org/10.1038/ja.2014.35 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative KEYWORDS * antifungal drug * copper ions * DLC * gatifloxacin * TLC
