Antenatal ureaplasma infection impairs development of the fetal ovine gut in an il-1-dependent manner
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Ureaplasma infection of the amniotic cavity is associated with adverse postnatal intestinal outcomes. We tested whether interleukin-1 (IL-1) signaling underlies intestinal pathology
following ureaplasma exposure in fetal sheep. Pregnant ewes received intra-amniotic injections of ureaplasma or culture media for controls at 3, 7, and 14 d before preterm delivery at 124 d
gestation (term 150 d). Intra-amniotic injections of recombinant human interleukin IL-1 receptor antagonist (rhIL-1ra) or saline for controls were given 3 h before and every 2 d after
Ureaplasma injection. Ureaplasma exposure caused fetal gut inflammation within 7 d with damaged villus epithelium and gut barrier loss. Proliferation, differentiation, and maturation of
enterocytes were significantly reduced after 7 d of ureaplasma exposure, leading to severe villus atrophy at 14 d. Inflammation, impaired development and villus atrophy of the fetal gut was
largely prevented by intra-uterine rhIL-1ra treatment. These data form the basis for a clinical understanding of the role of ureaplasma in postnatal intestinal pathologies. SIMILAR CONTENT
BEING VIEWED BY OTHERS PLACENTAL CLEARANCE NOT SYNTHESIS TEMPERS EXAGGERATED PRO-INFLAMMATORY CYTOKINE RESPONSE IN NEONATES EXPOSED TO CHORIOAMNIONITIS Article 11 June 2022 CHORIOAMNIONITIS
INDUCES HEPATIC INFLAMMATION AND TIME-DEPENDENT CHANGES OF THE ENTEROHEPATIC CIRCULATION IN THE OVINE FETUS Article Open access 14 May 2021 CHORIOAMNIONITIS INDUCES CHANGES IN OVINE
PULMONARY ENDOGENOUS EPITHELIAL STEM/PROGENITOR CELLS _IN UTERO_ Article 18 October 2020 INTRODUCTION Preterm birth is the primary cause of infant mortality and morbidity1 and the occurrence
of preterm birth has increased in the last decade.2 The majority of preterm births is associated with chorioamnionitis, an infection of the fetal membranes, as documented by histological
findings or culture.1 Ascending microbial invasion of the uterine cavity is considered to be the most frequent route of infection.3 Consistently, _Ureaplasma parvum_ (UP), a commensal of the
urogenital tract of humans is the most common microorganism associated with chorioamnionitis.4, 5 As the amniotic fluid along with organisms and inflammatory products are swallowed by the
fetus, the gastrointestinal tract is a possible route of fetal exposure that may result in fetal gut inflammation. Recently, an association was reported between ureaplasma colonization of
preterm infants and an increased incidence of necrotizing enterocolitis, a serious gastrointestinal disorder frequently affecting preterm neonates.6 However, the mechanisms by which
ureaplasma colonization can result in inflammation and injury of the preterm gut remains essentially unstudied. Using a translational chorioamnionitis model in fetal sheep, we recently
reported that intra-amniotic lipopolysaccharide resulted in fetal gut inflammation and subsequent altered maturation of the gut barrier and innate immune defences.7 Moreover, the harmful
inflammatory response of the fetal gut to lipopolysaccharide-induced chorioamnionitis could be largely reproduced by intra-amniotic interleukin-1 (IL-1) injection.8 We now hypothesize that
ureaplasma infection _in utero_ causes immune activation and concomitant disturbances of fetal gut development by IL-1-dependent mechanisms. We therefore administered intra-amniotic UP
_serovar 3_ for 3, 7, or 14 d before delivery in the presence or absence of the recombinant human IL-1 receptor antagonist (rhIL-1ra) to inhibit signaling by IL-1α and IL-1β9, 10 (see
Methods for experimental design). The effects of ureaplasma with or without rhIL-1ra on fetal gut inflammation, maturation, differentiation, and the intestinal barrier disturbance were
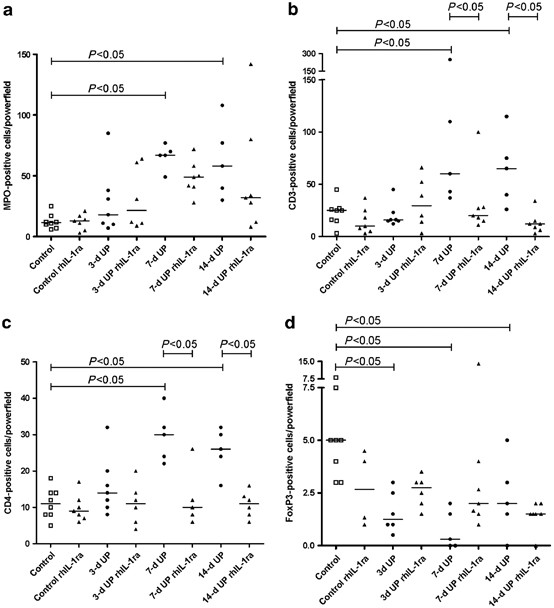
assessed. RESULTS INFLAMMATION The infection of the amniotic cavity by ureaplasma resulted in inflammation of the fetal gut. In control fetuses, a few myeloperoxidase-positive (MPO+) cells
were detected in the preterm gut and the number of infiltrating MPO+ cells did not change after 3-d exposure to ureaplasma (Figure 1a). However, the number of MPO+ cells increased
significantly in fetuses exposed to ureaplasma for 7 and 14 d when compared with controls of the same gestational age (GA). Intra-amniotic injection of rhIL-1ra before ureaplasma tended to
decrease the MPO+ cells in the fetal gut both at 7 and 14 d after ureaplasma exposure, but changes were not statistically significant (Figure 1a). MPO counts in control animals that received
culture medium remained unaltered by rhIL-1ra pre-treatment. Ureaplasma exposure induced the influx of CD3+ and CD4+ T cells, which largely paralleled the influx of MPO+ cells. CD3+ and
CD4+ T cells were increased in fetuses exposed to ureaplasma for 7and 14 d when compared with controls. This increased influx at 7 and 14 d after ureaplasma treatment was blocked by rhIL-1ra
(Figure 1b,c). We analyzed the expression of Foxp3, a prototypic marker of regulatory T cells which are essential for immune homeostasis in the intestinal tract.11, 12 When compared with
control animals, FoxP3-positive cells decreased 3, 7 and 14 d after UP exposure (Figure 1d). The depletion of FoxP3+ cells was not changed by rhIL-1ra exposure. TOLL-LIKE RECEPTOR MRNA
LEVELS The innate immune receptors responsible for sensing and signaling of _Ureaplasma spp_ include Toll-like receptor 2 (TLR2)–TLR613 and TLR2–TLR4.14 The mRNA levels of TLR2 and TLR6 tend
to increase in the fetal gut at 3 d after ureaplasma exposure, but changes were not statistically significant (Figure 2). TLR1, 2, 4, 6, and 9 mRNA levels were all increased at 7 d
post-ureaplasma exposure and these increased mRNA levels were inhibited by intra-amniotic rhIL-1ra injection (Figure 2). GUT DAMAGE AND MORPHOLOGICAL CHANGES We determined whether the
inflammatory response of the fetal gut after intra-amniotic ureaplasma exposure for 7 and 14 d resulted in subsequent intestinal damage and morphological changes. Hematoxylin and eosin
staining revealed that ureaplasma exposure for 7 and 14 d caused substantial injury to the enterocytes lining the tips of the villi (Figure 3). Damage to these vacuolated enterocytes, which
are characteristic for the immature gut,15, 16 was most prevalent in the 7-d-treated animals (Figure 3b). This sloughing of cells from the villi resulted in accumulation of luminal debris in
the proximity of the injured villi (Figure 3b). There was variation of both the intensity and location of the damage to the villi among the ureaplasma-exposed lambs. In a single animal,
there were patches where only a few cells were lost from the tips of the villi, whereas in adjacent regions, almost every villus was partially denuded of epithelium. Damage to the villus
epithelium and accumulation of luminal debris as seen after intra-amniotic ureaplasma injection was not prevented by blockage of IL-1 (Figure 3c). Besides loss of the villus epithelium,
significant villus shortening was observed in the 14-d ureaplasma group (Figure 3d,f). The reduced villus length in ureaplasma-exposed lambs was largely prevented by rhIL-1ra and comparable
with media-treated control fetuses (Figure 3e,f). The macroscopic damage to the villus epithelium was further substantiated by a disturbed distribution of the tight junction protein ZO-1
(_Zonula occludens_ protein 1), a critical component of the paracellular barrier. As reported previously, ZO-1 staining was fragmented in premature control animals.7, 8 Fetuses exposed to
ureaplasma for 7 or 14 d had a more fragmented ZO-1 distribution when compared with control animals, with the most severe disturbance in the 7-d group (Figure 4). The disturbed distribution
of ZO-1 in ureaplasma-exposed lambs was completely prevented by rhIL-1ra and was comparable with media-treated control fetuses (Figure 4). PROLIFERATION, DIFFERENTIATION, AND MATURATION OF
THE FETAL GUT In order to understand the mechanism behind the shortening of the villi, we analyzed the frequency and distribution of the four epithelial cell types that populate the fetal
intestinal crypts and villi, i.e., mucus-secreting goblet cells, hormone-secreting enteroendocrine cells, paneth cells, and enterocytes following ureaplasma treatment. Fetal ureaplasma
exposure did not change the numbers of goblet cells (Supplementary Figure S1 online) at any time point as assessed by periodic acid-Schiff (PAS) staining. However, in control fetuses, the
frequency of PAS-negative goblet cells was substantially higher compared with ureaplasma-treated fetuses, indicating that the mucin composition shifts from neutral (pink cells) to acidic
(blue cells) following ureaplasma exposure (Supplementary Figure S1 online). Paneth cells were not detectable after staining for PAS/Alcian blue or lysozyme. After 7 and 14 d of ureaplasma
exposure, endocrine cells were primarily located in the crypts (Figure 5b,d), which was in contrast to the normal distribution along the crypt–villus axis in media-treated fetuses (Figure
5a). In addition, a moderate decrease of endocrine cells was observed in lambs exposed to ureaplasma for 14 d (Figure 5d,e). RhIL-1ra did not prevent the change of PAS-positive into
PAS-negative cells (Supplementary Figure S1 online). However, the altered distribution of endocrine cells was restored by rhIL-1ra (Figure 5c,e). In contrast to goblet cells and endocrine
cells, a substantial decrease of enterocytes was observed in 14-d ureaplasma-treated animals when compared with control animals, and this effect was counterbalanced by rhIL-1ra (Figure 6).
In summary, these findings implicate that a reduced number of enterocytes is responsible for the villus shortening as seen in 14-d post-ureaplasma exposure. To gain insight whether the
reduced enterocyte number was caused by impaired proliferation, ileal tissue was stained for the proliferation marker KI67 and the mitotic marker Phospho-Histone H3. Fetuses exposed to
ureaplasma for 7 or 14 d had fewer proliferating crypt cells (Figure 7a–e) and mitotic cells (Figure 7f) when compared with controls. This decrease of proliferating and mitotic cells could
be blocked by rhIL-1ra treatment, although this effect on the number of mitotic cells in the 7-d ureaplasma group did not reach statistical significance. The mislocalization and small
decrease of endocrine cells and the reduced number of enterocytes prompted us to investigate the distribution of Kruppel-like factor 5 (KLF5), a transcription factor known to be involved in
maintenance of epithelial proliferation, differentiation, and cell positioning along the crypt radial axis.17 Consistent with previous reports,18 constitutive KLF5 expression was enriched in
intestinal epithelial cells located in the lower-to-middle crypt region (Figure 8a). In 7-d ureaplasma-treated animals, reduced numbers of KLF5+ cells were detected when compared with
media-treated controls, and these KLF5-expressing cells were restricted to the bottom of the crypts (Figure 8b). In lambs exposed to ureaplasma for 14 d, the majority of KLF5+ enterocytes
were identified at the bottom of the crypts, but in these animals, KLF5-expressing cells were also found in the upper part of the villus (Figure 8d). The altered KLF5 distribution following
ureaplasma exposure could be blocked by rhIL-1ra treatment (Figure 8c,e). Consistent with the reduced number of KLF5-expressing enterocytes in the 7-d ureaplasma-treated animals, intestinal
fatty-acid-binding protein (I-FABP) expression was reduced or absent in the villus epithelium in 7-d ureaplasma-treated fetuses and this reduced expression was counter-balanced in
rhIL-1ra-treated fetuses (Figure 9). As the loss of I-FABP expression in the gut following ureaplasma infection did not result in elevated fetal plasma levels for I-FABP (data not shown),
these data suggest that diminished differentiation, rather than cell death, is the cause of this phenotypic change of enterocytes. In line with this assumption, ureaplasma exposure did not
result in a higher number of apoptotic cells as assessed by caspase-3 staining (not shown). As the animals are infected not only for different lengths of time but also infected at different
stages of development, we tested the proliferation, differentiation, and maturation of the fetal ovine gut from 110–124 d GA in the absence of ureaplasma and or rhIL-1ra. As gut development
changed little over the period of 110–124 d of gestation (data not shown), the stage of development will have little influence on the response to ureaplasma or rhIL-1ra. DISCUSSION The most
important determinant of morbidity in prematurely born neonates is the immaturity of organs.19 Chorioamnionitis, which exposes the pulmonary and gastrointestinal tracts to microorganisms, is
one of the major causes of preterm birth.20, 21 Antenatal exposure of the lung to microorganisms changes early neonatal lung function and may alter long-term lung function and growth.22 In
addition, increasing evidence shows that chorioamnionitis interferes with intestinal development and is associated with adverse intestinal outcomes.6, 7, 8, 23, 24 This study reports the
novel finding that _in utero_ exposure to UP, the most common microorganism associated with chorioamnionitis, results in fetal gut inflammation, cellular damage to the villus epithelium,
accumulation of luminal debris, and disturbed distribution of the intracellular tight junctional ZO-1. Furthermore, ureaplasma colonization of the fetus led to substantial architectural
changes of the crypt–villus axis. In particular, proliferation, differentiation, and maturation of enterocytes were significantly perturbed as illustrated by decreased proliferation and a
strong reduction of KLF5- and I-FABP-expressing enterocytes. Interestingly, these early changes resulted in severe villus atrophy after ureaplasma exposure for 14 d. Importantly, our results
strongly link IL-1 activation with inflammation and underdevelopment of the intestine by demonstrating strong protective effects of prenatal rhIL-1ra administration in the course of
ureaplasma infection. In ureaplasma-exposed fetuses, rhIL-1ra treatment resulted in less intestinal inflammation, improvement of the barrier integrity, restored proliferation,
differentiation and maturation of enterocytes and prevention of villus atrophy. An increased pro-inflammatory status, disturbed barrier integrity and intestinal immaturity are considered to
underlie necrotizing enterocolitis pathology.25, 26, 27, 28 Therefore, our findings provide a mechanistic link for the clinical observation that ureaplasma colonization of the fetus is
associated with an increased risk of necrotizing enterocolitis development.6 Additionally, immaturity of intestinal epithelial cells and the decreased surface area of villi could result in
maldigestion and poor mucosal protection against colonizing bacteria in the postnatal period.29, 30 Different mechanisms may underlie the protective effects of rhIL-1ra in ureaplasma-treated
animals. First, our data suggest that rhIL-1ra-dependent recovery of the disturbed crypt–villus architecture, enterocyte immaturity and villus atrophy in ureaplasma-exposed fetuses are the
result of the recuperation of epithelial proliferation. Second, rhIL-1ra treatment prevented T-effector cell expansion and the associated detrimental inflammatory response in
ureaplasma-exposed fetuses, potentially by improvement of the apparent CD4+/FoxP3+ imbalance. Consistent with this theory, a regulatory T cell-to-T-effector cell imbalance has been recently
associated with an increased incidence of necrotizing enterocolitis, confirming that such a pro-inflammatory environment could lead to severe inflammation and damage of the preterm ileum.31
Third, rhIL-1ra completely prevented the ureaplasma-driven increase of TLRs, key receptors of the innate immune system which respond to a large group of pathogen-associated molecular
patterns, including lipoproteins from the _Ureaplasma spp_.13, 14 These data suggest that increased TLR signaling is causally linked with the detrimental inflammatory response in the
intestine after ureaplasma exposure. However, the proteins that mediate signaling from these receptors, such as MyD88 and IL-1 receptor-associated kinase-4, have also been shown to have
important roles in the response to microorganisms and warrant further investigation.32 Interestingly, the IL-1β protein was detected by an ovine IL-1β-specific enzyme-linked immunosorbent
assay (ELISA) in ileal tissue, whereas no IL-1β was detected in any of the amniotic fluid samples (data not shown). This result showed that the IL-1-dependent inflammatory and developmental
changes in the fetal gut following ureaplasma exposure were a direct response of the fetus rather then an indirect result of oral intake of IL-1-containing amniotic fluid. The increased
influx of MPO+-expressing cells, damage to the villus epithelium, and accumulation of luminal debris following intra-amniotic ureaplasma injection was not prevented by blockage of IL-1
signaling. This indicates that IL-1-independent mechanisms may also be responsible for some of the detrimental effects of ureaplasma colonization of the amniotic fluid. Although the net
result of an inflammatory response is the recruitment of inflammatory cells such as seen after 7 d after ureaplasma treatment, the current experimental set up does not allow us to study the
inflammatory response in more detail between 3 and 7 d after ureaplasma. Future studies have to take this into account in addition to the complex effects that the inhibition of IL-1 receptor
signaling may have on the multiple inflammatory events in the fetal ileum. Although our data suggest that rhIL-1ra has potential therapeutic use for ureaplasma-induced fetal gut
inflammation, it is currently unknown whether rhIL-1ra can be safely used in the human fetus. A limitation of the current study is that there is no insight into the effects of long-term
ureaplasma exposure on fetal gut inflammation and subsequent development. Future research is warranted to investigate whether fetal gut damage and interference with maturation further
progresses or resolves after longer ureaplasma exposure of the fetus. In addition, future studies should focus on the postnatal intestinal changes after intrauterine ureaplasma exposure and
the possible development of intestinal pathologies after birth. The important conclusion from the present study is that intrauterine ureaplasma exposure results in fetal gut inflammation,
intestinal injury and barrier loss, and impaired maturation. Moreover, these ureaplasma-induced effects on fetal gut inflammation and development are largely IL-1 dependent. Our data suggest
that administration of rhIL-1ra, which is used to treat immune disorders such as arthritis, could be a potential clinical tool to prevent the adverse intestinal outcomes upon birth after
chorioamnionitis. METHODS EXPERIMENTAL PROTOCOL. The animal studies were approved by the Animal Ethics Committee of The University of Western Australia, Perth, WA, Australia and the
Children's Hospital Medical Center, Cincinnati, OH. Ureaplasmas were grown and prepared for ultrasound-guided injections as reported previously.33 Time-mated Merino ewes with singleton
fetuses were randomly assigned to groups of five to seven animals to receive UP _serovar 3_ (2 × 107 colony-forming units (CFU)) or media control at 110, 117, or 121 d GA (term=150 d GA;
Figure 10). Colonization of the amniotic fluid with UP _3_ was confirmed in all ureaplasma-exposed lambs with >107 cfu ml−1 at delivery. Colonization was not detected in media controls.
Using the same ovine model, we previously demonstrated that injection of UP in the amniotic fluid resulted in chronic colonization of the amniotic fluid, the fetal lung and gut.34, 35 IL-1
signaling was inhibited with rhIL-1ra (anakinra (Kineret); Amgen, Thousand Oaks, CA). Intra-amniotic injections of 100 mg rhIL-1ra resuspended in 2 ml saline or the equivalent volume for
control animals were given 3 h before the intra-amniotic ureaplasma or media injections. Animals were then treated every 2 d with additional intra-amniotic injections of 100 mg rhIL-1ra or
saline (Figure 10). This dose and route of administration of rhIL-1ra completely inhibited intra-amniotic IL-1α-induced lung and systemic inflammation (reported in the online section of
Kallapur _et al._36). Intra-amniotic rhIL-1ra also blocked most of the inflammation and lung maturation induced by intra-amniotic lipopolysaccharide.36 At 124 d GA, the ewe was sedated (10
mg kg−1 ketamine, Parnell Labs, Alexandria, Australia; 0.02 mg kg−1 medetomidine, Pfizer Animal Health, West Ryde, Australia) before receiving a regional spinal anesthetic block (2%
lignocaine: 3 ml, 60 mg) and delivery of the preterm lamb by cesarean section. The lamb was immediately euthanized (100 mg kg−1 sodium pentobarbitone, Valabarb, Pitman-Moore, Australia) and
samples of the distal ileum were collected for snap freezing and fixation. ANTIBODIES. The following antibodies were used: rabbit antibodies against human Phospho-Histone H3 (Santa Cruz
Biotechnology, Santa Cruz, CA), MPO, CD3, lysozyme (all from Dakocytomation, Glostrup, Denmark), ZO-1 (Invitrogen, San Francisco, CA), and activated caspase-3 (Cell Signaling Technology,
Danvers, MA); rabbit anti-bovine SP-1 Chromogranin A (ImmunoStar, Hudson, WI); monoclonal antibodies against bovine CD4 (VMRD, Pullman, WA), human FoxP3 (eBioscience, San Diego, CA), and
human Ki-67 (Dakocytomation). Antibodies against I-FABP were kindly provided by the Department of surgery, Maastricht University Medical Center, the Netherlands headed by Prof. Steven Olde
Damink. A guinea pig antibody against human KLF5 was a kind gift from Dr. Jeffrey Whitsett from the Section of Neonatology, Perinatal and Pulmonary Biology, Cincinnati Children’s Hospital
Medical Center, Cincinnati, OH. Secondary antibodies, biotin-conjugated rabbit anti-mouse, goat anti-guinea pig or swine anti-rabbit, and Texas red-conjugated goat anti-rabbit were purchased
from Dakocytomation and peroxidase-conjugated goat anti-rabbit from Jackson (West Grove, PA). IMMUNOHISTOCHEMISTRY. Formalin-fixed gut tissue was embedded in paraffin and 3 μm sections were
used for immunohistochemistry. For detection of CD3, Ki-67, Phospho-Histone H3, lysozyme, Chromogranin A, Caspase-3 and KLF5-expressing cells, slides were boiled in 10 mM Na-citrate (pH
6.0) for 20 min. Endogenous peroxidase activity was blocked with 0.3% H2O2 in methanol. Slides were blocked with normal goat serum (MPO, FoxP3, Ki-67, Phospho-Histone H3, lysozyme,
Chromogranin A, Caspase-3, KLF5,) or bovine serum albumin (CD3, CD4) for 30 min at room temperature. Slides were incubated with the primary antibody of interest for 1 h at room temperature
(CD3, CD4, and MPO) or overnight at 4 °C (FoxP3, Ki-67, Phospho-Histone H3, lysozyme, Chromogranin A, Caspase-3, and KLF5). After washing using phosphate-buffered saline, sections were
incubated with the appropriate secondary-conjugated antibody. CD3 and FoxP3 antibodies were detected with the streptavidin-biotin system (Dakocytomation); antibodies against MPO and CD4 were
detected using a peroxidase-conjugated secondary antibody; antibodies against Ki-67, Phospho-Histone H3, lysozyme, Chromogranin A, Caspase-3, and KLF5 were detected using a
biotin-conjugated secondary antibody. Positive staining for MPO, CD3, and CD4 was visualized with 3-amino-9-ethylcarbazole (Sigma, St Louis, MO); nuclei were counterstained with
haematoxylin. Immunoreactivity for FoxP3, Ki-67, Phospho-Histone H3, lysozyme, Chromogranin A, Caspase-3, and KLF5 was visualized using nickel-DAB; and nuclei were counterstained with
nuclear fast red. The number of cells exhibiting immunostaining were counted per single (MPO, CD3, CD4, Phospho-Histone H3, Chromogranin A, × 200) or three high power fields (FoxP3, × 100).
The numbers of enterocytes were counted in sections stained for hematoxylin and eosin ( × 200). The immunohistochemical analysis was scored by three investigators who were blinded to the
experimental conditions. PAS/ALCIAN BLUE STAIN. De-waxed sections were immersed in Alcian Blue 8GX staining solution (pH 2.5; Richard Allan Scientific, MI) for 5 min. After washing in
distilled water, slides were oxidized in 1% periodic acid (Richard Allan Scientific) in water at room temperature for 5 min, washed in distilled water for 5 min, immersed in Schiff's
reagent (Richard Allan Scientific) for 5 min, and rinsed in tap water for 10 min. Following the three washing steps in distilled water, slides were counterstained in hematoxylin for 3 min
and dipped three times in phosphate-buffered saline. Then, slides were dehydrated and coverslipped. IMMUNOFLUORESENCE FOR ZO-1. Immunofluoresence was performed and interpreted as described
earlier.7 The ZO-1 distribution was recorded at a magnification of × 200 using the Metasystems Image Pro System (black and white charge-couple device camera; Metasystems, Sandhausen,
Germany) mounted on a Leica DM-RE fluorescence microscope (Leica, Wetzler, Germany). TLR MRNA QUANTITATION. Ileal total RNA was isolated and reverse transcribed into cDNA as previously
described.37 cDNA was used as a template with primers and Taqman probes (Applied Biosystems, Carlsbad, CA) specific to sheep sequences. The values for each TLR were normalized to the
internal RPS15 rRNA value. Data were expressed as fold increase over the control value. Primer sequences are provided in the Supplementary Table S1 online. ELISA. Differences of plasma
I-FABP levels were determined by ELISA to measure intestinal mucosal cell damage.38 For this purpose, a high-sensitive ELISA was used that was kindly provided by the Department of surgery,
Maastricht University Medical Center, the Netherlands headed by Prof. Steven Olde Damink. A similar protocol was used as previously described.39 STATISTICAL ANALYSIS. The number of cells
exhibiting immunostaining for the protein of interest were counted per high power field. Normality of all data obtained was evaluated by Kolmogorov–Smirnov test. Overall comparisons among
groups were performed by Kruskal–Wallis testing. Then individual between group comparisons were made using post-hoc Dunn’s multiple comparison testing. Data are presented as median and
range. Statistical calculations were made using SPSS 15.0 for Windows (SPSS, Chicago, IL) and differences were considered statistically significant at _P_<0.05. REFERENCES * Goldenberg,
R.L., Culhane, J.F., Iams, J.D. & Romero, R. Epidemiology and causes of preterm birth. _Lancet_ 371, 75–84 (2008). Article Google Scholar * Eichenwald, E.C. & Stark, A.R.
Management and outcomes of very low birth weight. _N. Engl. J. Med._ 358, 1700–1711 (2008). Article CAS Google Scholar * Goldenberg, R.L., Hauth, J.C. & Andrews, W.W. Intrauterine
infection and preterm delivery. _N. Engl. J. Med._ 342, 1500–1507 (2000). Article CAS Google Scholar * Oh, K.J. _et al_. Intraamniotic infection with genital mycoplasmas exhibits a more
intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. _Am. J. Obstet. Gynecol._ 203, 211 e211–211 e218
(2010). Article Google Scholar * Larsen, B. & Hwang, J. Mycoplasma, ureaplasma, and adverse pregnancy outcomes: a fresh look. _Infect. Dis. Obstet. Gynecol._ (article Id: 521921)
(2010). * Okogbule-Wonodi, A.C. _et al_. Necrotizing enterocolitis is associated with ureaplasma colonization in preterm infants. _Pediatr. Res._ 69, 442–447 (2011). Article Google Scholar
* Wolfs, T.G. _et al_. Endotoxin induced chorioamnionitis prevents intestinal development during gestation in fetal sheep. _PLoS One_ 4, e5837 (2009). Article Google Scholar * Wolfs,
T.G. _et al_. IL-1alpha mediated chorioamnionitis induces depletion of FoxP3+ cells and ileal inflammation in the ovine fetal gut. _PLoS One_ 6, e18355 (2011). Article CAS Google Scholar
* Fantuzzi, G. & Dinarello, C.A. The inflammatory response in interleukin-1 beta-deficient mice: comparison with other cytokine-related knock-out mice. _J. Leukoc. Biol._ 59, 489–493
(1996). Article CAS Google Scholar * Dinarello, C.A. Interleukin-1. _Cytokine Growth Factor Rev._ 8, 253–265 (1997). Article CAS Google Scholar * Veltkamp, C. _et al_. CD4+CD25+ cell
depletion from the normal CD4+ T cell pool prevents tolerance toward the intestinal flora and leads to chronic colitis in immunodeficient mice. _Inflamm. Bowel Dis._ 12, 437–446 (2006).
Article Google Scholar * Moes, N. _et al_. Reduced expression of FOXP3 and regulatory T-cell function in severe forms of early-onset autoimmune enteropathy. _Gastroenterology_ 139, 770–778
(2010). Article CAS Google Scholar * Shimizu, T., Kida, Y. & Kuwano, K. _Ureaplasma parvum_ lipoproteins, including MB antigen, activate NF-{kappa}B through TLR1, TLR2 and TLR6.
_Microbiology_ 154, 1318–1325 (2008). Article CAS Google Scholar * Peltier, M.R., Freeman, A.J., Mu, H.H. & Cole, B.C. Characterization of the macrophage-stimulating activity from
Ureaplasma urealyticum. _Am. J. Reprod. Immunol._ 57, 186–192 (2007). Article CAS Google Scholar * Verma, K.B. Development of mucosa of the human ileum. _J. Anat._ 128, 513–521 (1979).
CAS PubMed PubMed Central Google Scholar * Trahair, J. & Robinson, P. The development of the ovine small intestine. _Anat. Rec._ 214, 294–303 (1986). Article CAS Google Scholar *
McConnell, B.B. _et al_. Kruppel-like factor 5 is important for maintenance of crypt architecture and barrier function in mouse intestine. _Gastroenterology_ 141, 13002–13013 (2011). Article
Google Scholar * Conkright, M.D., Wani, M.A., Anderson, K.P. & Lingrel, J.B. A gene encoding an intestinal-enriched member of the Kruppel-like factor family expressed in intestinal
epithelial cells. _Nucleic Acids Res._ 27, 1263–1270 (1999). Article CAS Google Scholar * Hack, M. & Fanaroff, A.A. Outcomes of extremely immature infants--a perinatal dilemma. _N.
Engl. J. Med._ 329, 1649–1650 (1993). Article CAS Google Scholar * Been, J.V. _et al_. Histologic chorioamnionitis, fetal involvement, and antenatal steroids: effects on neonatal outcome
in preterm infants. _Am. J. Obstet. Gynecol._ 201, 587.e1–587.e8 (2009). Article Google Scholar * Thomas, W. & Speer, C.P. Chorioamnionitis: important risk factor or innocent bystander
for neonatal outcome? _Neonatology_ 99, 177–187 (2011). Article Google Scholar * Doyle, L.W. & Anderson, P.J. Pulmonary and neurological follow-up of extremely preterm infants.
_Neonatology_ 97, 388–394 (2010). Article Google Scholar * Andrews, W.W. _et al_. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other
markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. _Am. J. Obstet. Gynecol._ 195, 803–808 (2006). Article CAS Google Scholar * Giannone, P.J., Schanbacher,
B.L., Bauer, J.A. & Reber, K.M. Effects of prenatal lipopolysaccharide exposure on epithelial development and function in newborn rat intestine. _J. Pediatr. Gastroenterol. Nutr._ 43,
284–290 (2006). Article CAS Google Scholar * Lin, P.W. & Stoll, B.J. Necrotising enterocolitis. _Lancet_ 368, 1271–1283 (2006). Article Google Scholar * Neu, J. & Walker, W.A.
Necrotizing enterocolitis. _N. Engl. J. Med._ 364, 255–264 (2011). Article CAS Google Scholar * Anand, R.J., Leaphart, C.L., Mollen, K.P. & Hackam, D.J. The role of the intestinal
barrier in the pathogenesis of necrotizing enterocolitis. _Shock_ 27, 124–133 (2007). Article CAS Google Scholar * Emami, C.N. _et al_. Role of the host defense system and intestinal
microbial flora in the pathogenesis of necrotizing enterocolitis. _Surg. Infect. (Larchmt)_ 10, 407–417 (2009). Article Google Scholar * Wagner, C.L., Taylor, S.N. & Johnson, D. Host
factors in amniotic fluid and breast milk that contribute to gut maturation. _Clin. Rev. Allergy Immunol._ 34, 191–204 (2008). Article Google Scholar * Jiang, P. _et al_. Enteral feeding
_in utero_ induces marked intestinal structural and functional proteome changes in pig fetuses. _Pediatr. Res._ 69, 123–128 (2011). Article Google Scholar * Weitkamp, J.H. _et al_.
Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. _Gut_ ; e-pub ahead of print (2012). *
Nanthakumar, N. _et al_. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. _PLoS One_ 6, e17776 (2011). Article CAS
Google Scholar * Moss, T.J. _et al_. Experimental amniotic fluid infection in sheep: effects of _Ureaplasma parvum_ serovars 3 and 6 on preterm or term fetal sheep. _Am. J. Obstet.
Gynecol._ 198, 122e121–122e128 (2008). Article Google Scholar * Collins, J.J. _et al_. Inflammation in fetal sheep from intra-amniotic injection of _Ureaplasma parvum_. _Am. J. Physiol.
Lung Cell. Mol. Physiol._ 299, L852–L860 (2011). Article Google Scholar * Knox, C.L. _et al_. The severity of chorioamnionitis in pregnant sheep is associated with _in vivo_ variation of
the surface-exposed multiple-banded antigen/gene of _Ureaplasma parvum_. _Biol. Reprod._ 83, 415–426 (2010). Article CAS Google Scholar * Kallapur, S.G. _et al_. IL-1 mediates pulmonary
and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. _Am. J. Respir. Crit. Care Med._ 179, 955–961 (2009). Article CAS Google Scholar * Wolfs, T.G. _et
al_. Localization of the lipopolysaccharide recognition complex in the human healthy and inflamed premature and adult gut. _Inflamm. Bowel Dis._ 16, 68–75 (2010). Article Google Scholar *
Beuk, R.J. _et al_. Total warm ischemia and reperfusion impairs flow in all rat gut layers but increases leukocyte-vessel wall interactions in the submucosa only. _Ann. Surg._ 231, 96–104
(2000). Article CAS Google Scholar * Dello, S.A. _et al_. Total intermittent Pringle maneuver during liver resection can induce intestinal epithelial cell damage and endotoxemia. _PLoS
One_ 7, e30539 (2012). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We are grateful for the excellent technical assistance of Irma Tindemans (UM), Leon Janssen (UM),
and Kriti Puri (CCHMC). We thank Emma Sweeney (QUT) for assistance in culturing ureaplasmas from infected tissues. Funding: This study was supported by the National Institutes of Health
Grant HD-57869 (to SGK), the Dutch Scientific Research Organization Veni Grant 016.096.141 (to BWK), the Ter Meulen Fund of the Royal Netherlands Academy of Arts and Sciences (to TGAMW) and
the Research School for Oncology and Developmental Biology (GROW), Maastricht University. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Neonatology, Cincinnati Children’s
Hospital Medical Center, University of Cincinnati School of Medicine, Cincinnati, Ohio, USA T G A M Wolfs, S G Kallapur, G Thuijls & A H Jobe * Department of Pediatrics, Maastricht
University Medical Center (MUMC), School of Oncology and Developmental Biology (GROW), Maastricht, The Netherlands T G A M Wolfs, J J P Collins, E Kroon, J Spierings & B W Kramer *
School of Women’s and Infants Health, The University of Western Australia, Crawley, Western Australia, Australia S G Kallapur, I Nitsos, G R Polglase, J P Newnham & A H Jobe * Institute
of Health and Biomedical Innovation, Faculty of Science and Technology, Queensland University of Technology, Brisbane, Queensland, Australia C L Knox * The Ritchie Centre, Monash Institute
of Medical Research, Monash University, Clayton, Victoria, Australia G R Polglase * Division of Gastroenterology, Hepatology and Nutrition, Children’s Hospital Medical Center, University of
Cincinnati School of Medicine, Cincinnati, Ohio, USA N F Shroyer Authors * T G A M Wolfs View author publications You can also search for this author inPubMed Google Scholar * S G Kallapur
View author publications You can also search for this author inPubMed Google Scholar * C L Knox View author publications You can also search for this author inPubMed Google Scholar * G
Thuijls View author publications You can also search for this author inPubMed Google Scholar * I Nitsos View author publications You can also search for this author inPubMed Google Scholar *
G R Polglase View author publications You can also search for this author inPubMed Google Scholar * J J P Collins View author publications You can also search for this author inPubMed
Google Scholar * E Kroon View author publications You can also search for this author inPubMed Google Scholar * J Spierings View author publications You can also search for this author
inPubMed Google Scholar * N F Shroyer View author publications You can also search for this author inPubMed Google Scholar * J P Newnham View author publications You can also search for this
author inPubMed Google Scholar * A H Jobe View author publications You can also search for this author inPubMed Google Scholar * B W Kramer View author publications You can also search for
this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to B W Kramer. ETHICS DECLARATIONS COMPETING INTERESTS The authors declared no conflict of interest. ADDITIONAL
INFORMATION SUPPLEMENTARY MATERIAL is linked to the online version of the paper SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 (JPG 348 KB) SUPPLEMENTARY TABLE 1 (DOC 31 KB) POWERPOINT
SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 POWERPOINT SLIDE FOR FIG. 6 POWERPOINT
SLIDE FOR FIG. 7 POWERPOINT SLIDE FOR FIG. 8 POWERPOINT SLIDE FOR FIG. 9 POWERPOINT SLIDE FOR FIG. 10 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Wolfs, T., Kallapur, S., Knox, C. _et al._ Antenatal ureaplasma infection impairs development of the fetal ovine gut in an IL-1-dependent manner. _Mucosal Immunol_ 6, 547–556 (2013).
https://doi.org/10.1038/mi.2012.97 Download citation * Received: 03 May 2012 * Revised: 10 September 2012 * Accepted: 11 September 2012 * Published: 31 October 2012 * Issue Date: May 2013 *
DOI: https://doi.org/10.1038/mi.2012.97 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
