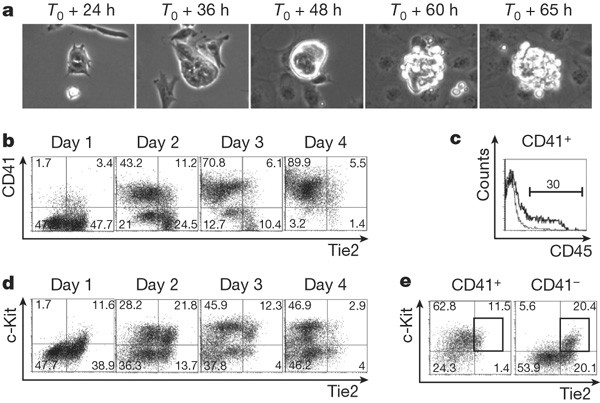
The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT It has been proposed that during embryonic development haematopoietic cells arise from a mesodermal progenitor with both endothelial and haematopoietic potential called the
haemangioblast1,2. A conflicting theory instead associates the first haematopoietic cells with a phenotypically differentiated endothelial cell that has haematopoietic potential (that is, a
haemogenic endothelium)3,4,5. Support for the haemangioblast concept was initially provided by the identification during mouse embryonic stem cell differentiation of a clonal precursor, the
blast colony-forming cell (BL-CFC), which gives rise to blast colonies with both endothelial and haematopoietic components6,7. Although recent studies have now provided evidence for the
presence of this bipotential precursor _in vivo_8,9, the precise mechanism for generation of haematopoietic cells from the haemangioblast still remains completely unknown. Here we
demonstrate that the haemangioblast generates haematopoietic cells through the formation of a haemogenic endothelium intermediate, providing the first direct link between these two precursor
populations. The cell population containing the haemogenic endothelium is transiently generated during BL-CFC development. This cell population is also present in gastrulating mouse embryos
and generates haematopoietic cells on further culture. At the molecular level, we demonstrate that the transcription factor Tal1 (also known as Scl; ref. 10) is indispensable for the
establishment of this haemogenic endothelium population whereas the core binding factor Runx1 (also known as AML1; ref. 11) is critical for generation of definitive haematopoietic cells from
haemogenic endothelium. Together our results merge the two a priori conflicting theories on the origin of haematopoietic development into a single linear developmental process. Access
through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal
Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may
be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
SIMILAR CONTENT BEING VIEWED BY OTHERS IDENTIFYING A NOVEL ROLE FOR THE MASTER REGULATOR _TAL1_ IN THE ENDOTHELIAL TO HEMATOPOIETIC TRANSITION Article Open access 10 October 2022 CD32
CAPTURES COMMITTED HAEMOGENIC ENDOTHELIAL CELLS DURING HUMAN EMBRYONIC DEVELOPMENT Article Open access 09 April 2024 MAPPING HUMAN HAEMATOPOIETIC STEM CELLS FROM HAEMOGENIC ENDOTHELIUM TO
BIRTH Article 13 April 2022 REFERENCES * Sabin, F. R. Studies on the origin of blood vessels and of red corpuscules as seen in the living blastoderm of the chick during the second day of
incubation: contributions to embryology. _Contrib. Embryol._ 9, 213–262 (1920) Google Scholar * Murray, P. D. F. The development _in vitro_ of the blood of the early chick embryo. _Proc. R.
Soc. Lond._ 11, 497–521 (1932) ADS Google Scholar * Jaffredo, T., Gautier, R., Eichmann, A. & Dieterlen-Lievre, F. Intraaortic hemopoietic cells are derived from endothelial cells
during ontogeny. _Development_ 125, 4575–4583 (1998) Article CAS Google Scholar * Nishikawa, S. I. et al. _In vitro_ generation of lymphohematopoietic cells from endothelial cells
purified from murine embryos. _Immunity_ 8, 761–769 (1998) Article CAS Google Scholar * North, T. E. et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the
midgestation mouse embryo. _Immunity_ 16, 661–672 (2002) Article CAS Google Scholar * Kennedy, M. et al. A common precursor for primitive erythropoiesis and definitive haematopoiesis.
_Nature_ 386, 488–493 (1997) Article ADS CAS Google Scholar * Choi, K., Kennedy, M., Kazarov, A., Papadimitriou, J. C. & Keller, G. A common precursor for hematopoietic and
endothelial cells. _Development_ 125, 725–732 (1998) Article CAS Google Scholar * Huber, T. L., Kouskoff, V., Fehling, H. J., Palis, J. & Keller, G. Haemangioblast commitment is
initiated in the primitive streak of the mouse embryo. _Nature_ 432, 625–630 (2004) Article ADS CAS Google Scholar * Vogeli, K. M., Jin, S. W., Martin, G. R. & Stainier, D. Y. A
common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. _Nature_ 443, 337–339 (2006) Article ADS CAS Google Scholar * Porcher, C. et al. The T cell
leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. _Cell_ 86, 47–57 (1996) Article CAS Google Scholar * Okuda, T., van Deursen, J., Hiebert, S. W.,
Grosveld, G. & Downing, J. R. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. _Cell_ 84, 321–330 (1996)
Article CAS Google Scholar * Faloon, P. et al. Basic fibroblast growth factor positively regulates hematopoietic development. _Development_ 127, 1931–1941 (2000) Article CAS Google
Scholar * Dumont, D. J., Yamaguchi, T. P., Conlon, R. A., Rossant, J. & Breitman, M. L. _tek_, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial
cells and their presumptive precursors. _Oncogene_ 7, 1471–1480 (1992) CAS PubMed Google Scholar * Ferkowicz, M. J. et al. CD41 expression defines the onset of primitive and definitive
hematopoiesis in the murine embryo. _Development_ 130, 4393–4403 (2003) Article CAS Google Scholar * Mikkola, H. K., Fujiwara, Y., Schlaeger, T. M., Traver, D. & Orkin, S. H.
Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. _Blood_ 101, 508–516 (2003) Article CAS Google Scholar * Li, W., Ferkowicz, M. J., Johnson, S. A.,
Shelley, W. C. & Yoder, M. C. Endothelial cells in the early murine yolk sac give rise to CD41-expressing hematopoietic cells. _Stem Cells Dev._ 14, 44–54 (2005) Article Google Scholar
* Newman, P. J. et al. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. _Science_ 247, 1219–1222 (1990) Article ADS CAS Google Scholar
* Hirai, H. et al. Hemogenic and nonhemogenic endothelium can be distinguished by the activity of fetal liver kinase (Flk)-1 promoter/enhancer during mouse embryogenesis. _Blood_ 101,
886–893 (2003) Article CAS Google Scholar * Hallmann, R., Mayer, D. N., Berg, E. L., Broermann, R. & Butcher, E. C. Novel mouse endothelial cell surface marker is suppressed during
differentiation of the blood brain barrier. _Dev. Dyn._ 202, 325–332 (1995) Article CAS Google Scholar * Young, P. E., Baumhueter, S. & Lasky, L. A. The sialomucin CD34 is expressed
on hematopoietic cells and blood vessels during murine development. _Blood_ 85, 96–105 (1995) Article CAS Google Scholar * Li, D. Y. et al. Defective angiogenesis in mice lacking
endoglin. _Science_ 284, 1534–1537 (1999) Article ADS CAS Google Scholar * Fong, G. H., Rossant, J., Gertsenstein, M. & Breitman, M. L. Role of the Flt-1 receptor tyrosine kinase in
regulating the assembly of vascular endothelium. _Nature_ 376, 66–70 (1995) Article ADS CAS Google Scholar * Breier, G. et al. Molecular cloning and expression of murine vascular
endothelial-cadherin in early stage development of cardiovascular system. _Blood_ 87, 630–641 (1996) Article CAS Google Scholar * Mukouyama, Y. et al. The AML1 transcription factor
functions to develop and maintain hematogenic precursor cells in the embryonic aorta–gonad–mesonephros region. _Dev. Biol._ 220, 27–36 (2000) Article CAS Google Scholar * Fehling, H. J.
et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. _Development_ 130, 4217–4227 (2003) Article CAS Google Scholar *
Palis, J., Robertson, S., Kennedy, M., Wall, C. & Keller, G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. _Development_ 126, 5073–5084
(1999) Article CAS Google Scholar * Lacaud, G. et al. Runx1 is essential for hematopoietic commitment at the hemangioblast stage of development _in vitro_ . _Blood_ 100, 458–466 (2002)
Article CAS Google Scholar * Voyta, J. C., Via, D. P., Butterfield, C. E. & Zetter, B. R. Identification and isolation of endothelial cells based on their increased uptake of
acetylated-low density lipoprotein. _J. Cell Biol._ 99, 2034–2040 (1984) Article CAS Google Scholar * D’Souza, S. L., Elefanty, A. G. & Keller, G. SCL/Tal-1 is essential for
hematopoietic commitment of the hemangioblast but not for its development. _Blood_ 105, 3862–3870 (2005) Article Google Scholar * Kyba, M., Perlingeiro, R. C. & Daley, G. Q. HoxB4
confers definitive lymphoid–myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. _Cell_ 109, 29–37 (2002) Article CAS Google Scholar Download
references ACKNOWLEDGEMENTS We thank K. Labib, C. Miller and T. Somervaille for critical reading of the manuscript, J. Barry and M. Hughes for cell sorting, S. Bagley for help with the
time-lapse photography, G. Ashton for help with preparation of sections, and L. Gautreau for advice with the OP9 cultures. Cancer Research UK supported this work. AUTHOR CONTRIBUTIONS P.S.
designed and performed experiments, and analysed the data. C.S. performed experiments. T.A. designed research. C.L., V.K. and G.L. designed the research, performed experiments, analysed the
data and wrote the paper. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Cancer Research UK Stem Cell Biology Group,, Christophe Lancrin, Patrycja Sroczynska, Catherine Stephenson &
Georges Lacaud * Cancer Research UK Structural Cell Biology Group,, Terry Allen * Cancer Research UK Stem Cell Haematopoiesis Group, Paterson Institute for Cancer Research, University of
Manchester, Wilmslow road, Manchester M20 4BX, UK , Valerie Kouskoff Authors * Christophe Lancrin View author publications You can also search for this author inPubMed Google Scholar *
Patrycja Sroczynska View author publications You can also search for this author inPubMed Google Scholar * Catherine Stephenson View author publications You can also search for this author
inPubMed Google Scholar * Terry Allen View author publications You can also search for this author inPubMed Google Scholar * Valerie Kouskoff View author publications You can also search for
this author inPubMed Google Scholar * Georges Lacaud View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Georges
Lacaud. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES This file contains Supplementary Figures 1-11 with Legends (PDF 4489 kb) SUPPLEMENTARY VIDEO 1 Supplementary Video 1 shows the
generation of non-adherent cells from tight adherent structures observed during blast cultures established with Flk1+ wild type cells. (MOV 5810 kb) SUPPLEMENTARY VIDEO 2 Supplementary Video
2 shows in the circle the generation of a blast colony from one Flk1+ cell through an intermediate stage corresponding to a cluster of tightly associated cells. Generation of blast colonies
following aggregation of cells of different origins is also observed. (MOV 4873 kb) SUPPLEMENTARY VIDEO 3 Supplementary Video 3 shows the absence of generation of non adherent cells from
tight adherent structures observed during blast cultures established with Flk1+ Runx1-/- cells. (MOV 3746 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2
POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lancrin, C., Sroczynska, P., Stephenson, C. _et
al._ The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. _Nature_ 457, 892–895 (2009). https://doi.org/10.1038/nature07679 Download citation * Received:
08 August 2008 * Accepted: 01 December 2008 * Published: 01 February 2009 * Issue Date: 12 February 2009 * DOI: https://doi.org/10.1038/nature07679 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
