Isolation and characterization of a bat sars-like coronavirus that uses the ace2 receptor
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The 2002–3 pandemic caused by severe acute respiratory syndrome coronavirus (SARS-CoV) was one of the most significant public health events in recent history1. An ongoing outbreak
of Middle East respiratory syndrome coronavirus2 suggests that this group of viruses remains a key threat and that their distribution is wider than previously recognized. Although bats have
been suggested to be the natural reservoirs of both viruses3,4,5, attempts to isolate the progenitor virus of SARS-CoV from bats have been unsuccessful. Diverse SARS-like coronaviruses
(SL-CoVs) have now been reported from bats in China, Europe and Africa5,6,7,8, but none is considered a direct progenitor of SARS-CoV because of their phylogenetic disparity from this virus
and the inability of their spike proteins to use the SARS-CoV cellular receptor molecule, the human angiotensin converting enzyme II (ACE2)9,10. Here we report whole-genome sequences of two
novel bat coronaviruses from Chinese horseshoe bats (family: Rhinolophidae) in Yunnan, China: RsSHC014 and Rs3367. These viruses are far more closely related to SARS-CoV than any previously
identified bat coronaviruses, particularly in the receptor binding domain of the spike protein. Most importantly, we report the first recorded isolation of a live SL-CoV (bat SL-CoV-WIV1)
from bat faecal samples in Vero E6 cells, which has typical coronavirus morphology, 99.9% sequence identity to Rs3367 and uses ACE2 from humans, civets and Chinese horseshoe bats for cell
entry. Preliminary _in vitro_ testing indicates that WIV1 also has a broad species tropism. Our results provide the strongest evidence to date that Chinese horseshoe bats are natural
reservoirs of SARS-CoV, and that intermediate hosts may not be necessary for direct human infection by some bat SL-CoVs. They also highlight the importance of pathogen-discovery programs
targeting high-risk wildlife groups in emerging disease hotspots as a strategy for pandemic preparedness. SIMILAR CONTENT BEING VIEWED BY OTHERS BAT CORONAVIRUSES RELATED TO SARS-COV-2 AND
INFECTIOUS FOR HUMAN CELLS Article 16 February 2022 ACE2 RECEPTOR USAGE REVEALS VARIATION IN SUSCEPTIBILITY TO SARS-COV AND SARS-COV-2 INFECTION AMONG BAT SPECIES Article 01 March 2021 CLOSE
RELATIVES OF MERS-COV IN BATS USE ACE2 AS THEIR FUNCTIONAL RECEPTORS Article Open access 07 December 2022 MAIN The 2002–3 pandemic of SARS1 and the ongoing emergence of the Middle East
respiratory syndrome coronavirus (MERS-CoV)2 demonstrate that CoVs are a significant public health threat. SARS-CoV was shown to use the human ACE2 molecule as its entry receptor, and this
is considered a hallmark of its cross-species transmissibility11. The receptor binding domain (RBD) located in the amino-terminal region (amino acids 318–510) of the SARS-CoV spike (S)
protein is directly involved in binding to ACE2 (ref. 12). However, despite phylogenetic evidence that SARS-CoV evolved from bat SL-CoVs, all previously identified SL-CoVs have major
sequence differences from SARS-CoV in the RBD of their S proteins, including one or two deletions6,9. Replacing the RBD of one SL-CoV S protein with SARS-CoV S conferred the ability to use
human ACE2 and replicate efficiently in mice9,13. However, to date, no SL-CoVs have been isolated from bats, and no wild-type SL-CoV of bat origin has been shown to use ACE2. We conducted a
12-month longitudinal survey (April 2011–September 2012) of SL-CoVs in a colony of _Rhinolophus sinicus_ at a single location in Kunming, Yunnan Province, China (Extended Data Table 1). A
total of 117 anal swabs or faecal samples were collected from individual bats using a previously published method5,14. A one-step reverse transcription (RT)-nested PCR was conducted to
amplify the RNA-dependent RNA polymerase (RdRP) motifs A and C, which are conserved among alphacoronaviruses and betacoronaviruses15. Twenty-seven of the 117 samples (23%) were classed as
positive by PCR and subsequently confirmed by sequencing. The species origin of all positive samples was confirmed to be _R. sinicus_ by cytochrome b sequence analysis, as described
previously16. A higher prevalence was observed in samples collected in October (30% in 2011 and 48.7% in 2012) than those in April (7.1% in 2011) or May (7.4% in 2012) (Extended Data Table
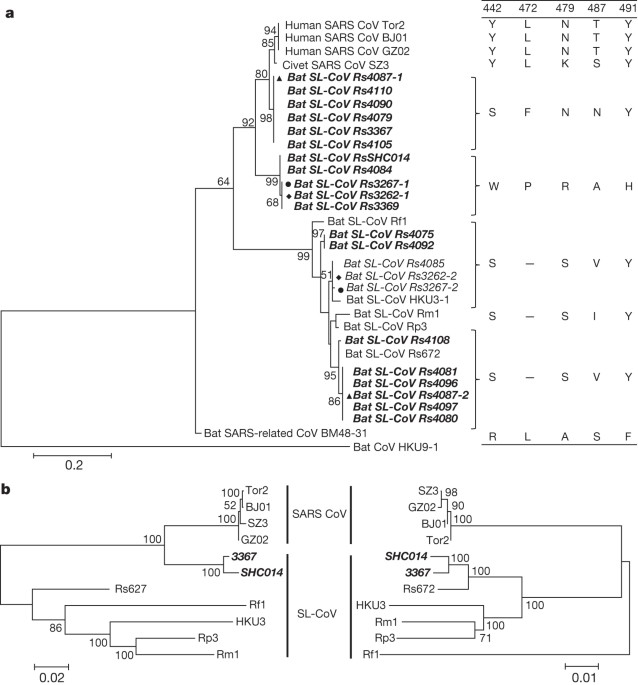
1). Analysis of the S protein RBD sequences indicated the presence of seven different strains of SL-CoVs (Fig. 1a and Extended Data Figs 1 and 2). In addition to RBD sequences, which closely
matched previously described SL-CoVs (Rs672, Rf1 and HKU3)5,8,17,18, two novel strains (designated SL-CoV RsSHC014 and Rs3367) were discovered. Their full-length genome sequences were
determined, and both were found to be 29,787 base pairs in size (excluding the poly(A) tail). The overall nucleotide sequence identity of these two genomes with human SARS-CoV (Tor2 strain)
is 95%, higher than that observed previously for bat SL-CoVs in China (88–92%)5,8,17,18 or Europe (76%)6 (Extended Data Table 2 and Extended Data Figs 3 and 4). Higher sequence identities
were observed at the protein level between these new SL-CoVs and SARS-CoVs (Extended Data Tables 3 and 4). To understand the evolutionary origin of these two novel SL-CoV strains, we
conducted recombination analysis with the Recombination Detection Program 4.0 package19 using available genome sequences of bat SL-CoV strains (Rf1, Rp3, Rs672, Rm1, HKU3 and BM48-31) and
human and civet representative SARS-CoV strains (BJ01, SZ3, Tor2 and GZ02). Three breakpoints were detected with strong _P_ values (<10−20) and supported by similarity plot and bootscan
analysis (Extended Data Fig. 5a, b). Breakpoints were located at nucleotides 20,827, 26,553 and 28,685 in the Rs3367 (and RsSHC014) genome, and generated recombination fragments covering
nucleotides 20,827–26,533 (5,727 nucleotides) (including partial open reading frame (ORF) 1b, full-length S, ORF3, E and partial M gene) and nucleotides 26,534–28,685 (2,133 nucleotides)
(including partial ORF M, full-length ORF6, ORF7, ORF8 and partial N gene). Phylogenetic analysis using the major and minor parental regions suggested that Rs3367, or RsSHC014, is the
descendent of a recombination of lineages that ultimately lead to SARS-CoV and SL-CoV Rs672 (Fig. 1b). The most notable sequence differences between these two new SL-CoVs and previously
identified SL-CoVs is in the RBD regions of their S proteins. First, they have higher amino acid sequence identity to SARS-CoV (85% and 96% for RsSHC014 and Rs3367, respectively). Second,
there are no deletions and they have perfect sequence alignment with the SARS-CoV RBD region (Extended Data Figs 1 and 2). Structural and mutagenesis studies have previously identified five
key residues (amino acids 442, 472, 479, 487 and 491) in the RBD of the SARS-CoV S protein that have a pivotal role in receptor binding20,21. Although all five residues in the RsSHC014 S
protein were found to be different from those of SARS-CoV, two of the five residues in the Rs3367 RBD were conserved (Fig. 1 and Extended Data Fig. 1). Despite the rapid accumulation of bat
CoV sequences in the last decade, there has been no report of successful virus isolation6,22,23. We attempted isolation from SL-CoV PCR-positive samples. Using an optimized protocol and Vero
E6 cells, we obtained one isolate which caused cytopathic effect during the second blind passage. Purified virions displayed typical coronavirus morphology under electron microscopy (Fig.
2). Sequence analysis using a sequence-independent amplification method14 to avoid PCR-introduced contamination indicated that the isolate was almost identical to Rs3367, with 99.9%
nucleotide genome sequence identity and 100% amino acid sequence identity for the S1 region. The new isolate was named SL-CoV-WIV1. To determine whether WIV1 can use ACE2 as a cellular entry
receptor, we conducted virus infectivity studies using HeLa cells expressing or not expressing ACE2 from humans, civets or Chinese horseshoe bats. We found that WIV1 is able to use ACE2 of
different origins as an entry receptor and replicated efficiently in the ACE2-expressing cells (Fig. 3). This is, to our knowledge, the first identification of a wild-type bat SL-CoV capable
of using ACE2 as an entry receptor. To assess its cross-species transmission potential, we conducted infectivity assays in cell lines from a range of species. Our results (Fig. 4 and
Extended Data Table 5) indicate that bat SL-CoV-WIV1 can grow in human alveolar basal epithelial (A549), pig kidney 15 (PK-15) and _Rhinolophus sinicus_ kidney (RSKT) cell lines, but not in
human cervix (HeLa), Syrian golden hamster kidney (BHK21), _Myotis davidii_ kidney (BK), _Myotis chinensis_ kidney (MCKT), _Rousettus leschenaulti_ kidney (RLK) or _Pteropus alecto_ kidney
(PaKi) cell lines. Real-time RT–PCR indicated that WIV1 replicated much less efficiently in A549, PK-15 and RSKT cells than in Vero E6 cells (Fig. 4). To assess the cross-neutralization
activity of human SARS-CoV sera against WIV1, we conducted serum-neutralization assays using nine convalescent sera from SARS patients collected in 2003. The results showed that seven of
these were able to completely neutralize 100 tissue culture infectious dose 50 (TCID50) WIV1 at dilutions of 1:10 to 1:40, further confirming the close relationship between WIV1 and
SARS-CoV. Our findings have important implications for public health. First, they provide the clearest evidence yet that SARS-CoV originated in bats. Our previous work provided phylogenetic
evidence of this5, but the lack of an isolate or evidence that bat SL-CoVs can naturally infect human cells, until now, had cast doubt on this hypothesis. Second, the lack of capacity of
SL-CoVs to use of ACE2 receptors has previously been considered as the key barrier for their direct spillover into humans, supporting the suggestion that civets were intermediate hosts for
SARS-CoV adaptation to human transmission during the SARS outbreak24. However, the ability of SL-CoV-WIV1 to use human ACE2 argues against the necessity of this step for SL-CoV-WIV1 and
suggests that direct bat-to-human infection is a plausible scenario for some bat SL-CoVs. This has implications for public health control measures in the face of potential spillover of a
diverse and growing pool of recently discovered SARS-like CoVs with a wide geographic distribution. Our findings suggest that the diversity of bat CoVs is substantially higher than that
previously reported. In this study we were able to demonstrate the circulation of at least seven different strains of SL-CoVs within a single colony of _R. sinicus_ during a 12-month period.
The high genetic diversity of SL-CoVs within this colony was mirrored by high phenotypic diversity in the differential use of ACE2 by different strains. It would therefore not be surprising
if further surveillance reveals a broad diversity of bat SL-CoVs that are able to use ACE2, some of which may have even closer homology to SARS-CoV than SL-CoV-WIV1. Our results—in addition
to the recent demonstration of MERS-CoV in a Saudi Arabian bat25, and of bat CoVs closely related to MERS-CoV in China, Africa, Europe and North America3,26,27—suggest that bat
coronaviruses remain a substantial global threat to public health. Finally, this study demonstrates the public health importance of pathogen discovery programs targeting wildlife that aim to
identify the ‘known unknowns’—previously unknown viral strains closely related to known pathogens. These programs, focused on specific high-risk wildlife groups and hotspots of disease
emergence, may be a critical part of future global strategies to predict, prepare for, and prevent pandemic emergence28. METHODS SUMMARY Throat and faecal swabs or fresh faecal samples were
collected in viral transport medium as described previously14. All PCR was conducted with the One-Step RT–PCR kit (Invitrogen). Primers targeting the highly conserved regions of the RdRP
gene were used for detection of all alphacoronaviruses and betacoronaviruses as described previously15. Degenerate primers were designed on the basis of all available genomic sequences of
SARS-CoVs and SL-CoVs and used for amplification of the RBD sequences of S genes or full-length genomic sequences. Degenerate primers were used for amplification of the bat _ACE2_ gene as
described previously29. PCR products were gel purified and cloned into pGEM-T Easy Vector (Promega). At least four independent clones were sequenced to obtain a consensus sequence.
PCR-positive faecal samples (in 200 μl buffer) were gradient centrifuged at 3,000–12,000_g_ and supernatant diluted at 1:10 in DMEM before being added to Vero E6 cells. After incubation at
37 °C for 1 h, inocula were removed and replaced with fresh DMEM with 2% FCS. Cells were incubated at 37 °C and checked daily for cytopathic effect. Cell lines from different origins were
grown on coverslips in 24-well plates and inoculated with the novel SL-CoV at a multiplicity of infection of 10. Virus replication was detected at 24 h after infection using rabbit
antibodies against the SL-CoV Rp3 nucleocapsid protein followed by Cy3-conjugated goat anti-rabbit IgG. ONLINE METHODS SAMPLING Bats were trapped in their natural habitat as described
previously5. Throat and faecal swab samples were collected in viral transport medium (VTM) composed of Hank’s balanced salt solution, pH 7.4, containing BSA (1%), amphotericin (15 μg ml−1),
penicillin G (100 U ml−1) and streptomycin (50 μg ml−1). To collect fresh faecal samples, clean plastic sheets measuring 2.0 by 2.0 m were placed under known bat roosting sites at about
18:00 h each evening. Relatively fresh faecal samples were collected from sheets at approximately 05:30–06:00 the next morning and placed in VTM. Samples were transported to the laboratory
and stored at −80 °C until use. All animals trapped for this study were released back to their habitat after sample collection. All sampling processes were performed by veterinarians with
approval from Animal Ethics Committee of the Wuhan Institute of Virology (WIVH05210201) and EcoHealth Alliance under an inter-institutional agreement with University of California, Davis (UC
Davis protocol no. 16048). RNA EXTRACTION, PCR AND SEQUENCING RNA was extracted from 140 μl of swab or faecal samples with a Viral RNA Mini Kit (Qiagen) following the manufacturer’s
instructions. RNA was eluted in 60 μl RNAse-free buffer (buffer AVE, Qiagen), then aliquoted and stored at −80 °C. One-step RT–PCR (Invitrogen) was used to detect coronavirus sequences as
described previously15. First round PCR was conducted in a 25-μl reaction mix containing 12.5 μl PCR 2× reaction mix buffer, 10 pmol of each primer, 2.5 mM MgSO4, 20 U RNase inhibitor, 1 μl
SuperScript III/ Platinum Taq Enzyme Mix and 5 μl RNA. Amplification of the RdRP-gene fragment was performed as follows: 50 °C for 30 min, 94 °C for 2 min, followed by 40 cycles consisting
of 94 °C for 15 s, 62 °C for 15 s, 68 °C for 40 s, and a final extension of 68 °C for 5 min. Second round PCR was conducted in a 25-μl reaction mix containing 2.5 μl PCR reaction buffer, 5
pmol of each primer, 50 mM MgCl2, 0.5 mM dNTP, 0.1 μl Platinum Taq Enzyme (Invitrogen) and 1 μl first round PCR product. The amplification of RdRP-gene fragment was performed as follows: 94
°C for 5 min followed by 35 cycles consisting of 94 °C for 30 s, 52 °C for 30 s, 72 °C for 40 s, and a final extension of 72 °C for 5 min. To amplify the RBD region, one-step RT–PCR was
performed with primers designed based on available SARS-CoV or bat SL-CoVs (first round PCR primers; F, forward; R, reverse: CoVS931F-5′-VWGADGTTGTKAGRTTYCCT-3′ and
CoVS1909R-5′-TAARACAVCCWGCYTGWGT-3′; second PCR primers: CoVS951F-5′-TGTKAGRTTYCCTAAYATTAC-3′ and CoVS1805R-5′-ACATCYTGATANARAACAGC-3′). First-round PCR was conducted in a 25-μl reaction mix
as described above except primers specific for the S gene were used. The amplification of the RBD region of the S gene was performed as follows: 50 °C for 30 min, 94 °C for 2 min, followed
by 35 cycles consisting of 94 °C for 15 s, 43 °C for 15 s, 68 °C for 90 s, and a final extension of 68 °C for 5 min. Second-round PCR was conducted in a 25-μl reaction mix containing 2.5 μl
PCR reaction buffer, 5 pmol of each primer, 50 mM MgCl2, 0.5 mM dNTP, 0.1 μl Platinum Taq Enzyme (Invitrogen) and 1 μl first round PCR product. Amplification was performed as follows: 94 °C
for 5 min followed by 40 cycles consisting of 94 °C for 30 s, 41 °C for 30 s, 72 °C for 60 s, and a final extension of 72 °C for 5 min. PCR products were gel purified and cloned into pGEM-T
Easy Vector (Promega). At least four independent clones were sequenced to obtain a consensus sequence for each of the amplified regions. SEQUENCING FULL-LENGTH GENOMES Degenerate coronavirus
primers were designed based on all available SARS-CoV and bat SL-CoV sequences in GenBank and specific primers were designed from genome sequences generated from previous rounds of
sequencing in this study (primer sequences will be provided upon request). All PCRs were conducted using the One-Step RT–PCR kit (Invitrogen). The 5′ and 3′ genomic ends were determined
using the 5′ or 3′ RACE kit (Roche), respectively. PCR products were gel purified and sequenced directly or following cloning into pGEM-T Easy Vector (Promega). At least four independent
clones were sequenced to obtain a consensus sequence for each of the amplified regions and each region was sequenced at least twice. SEQUENCE ANALYSIS AND DATABANK ACCESSION NUMBERS Routine
sequence management and analysis was carried out using DNAStar or Geneious. Sequence alignment and editing was conducted using ClustalW, BioEdit or GeneDoc. Maximum Likelihood phylogenetic
trees based on the protein sequences were constructed using a Poisson model with bootstrap values determined by 1,000 replicates in the MEGA5 software package. Sequences obtained in this
study have been deposited in GenBank as follows (accession numbers given in parenthesis): full-length genome sequence of SL-CoV RsSHC014 and Rs3367 (KC881005, KC881006); full-length sequence
of WIV1 S (KC881007); RBD (KC880984-KC881003); ACE2 (KC8810040). SARS-CoV sequences used in this study: human SARS-CoV strains Tor2 (AY274119), BJ01 (AY278488), GZ02 (AY390556) and civet
SARS-CoV strain SZ3 (AY304486). Bat coronavirus sequences used in this study: Rs672 (FJ588686), Rp3 (DQ071615), Rf1 (DQ412042), Rm1 (DQ412043), HKU3-1 (DQ022305), BM48-31 (NC_014470), HKU9-1
(NC_009021), HKU4 (NC_009019), HKU5 (NC_009020), HKU8 (DQ249228), HKU2 (EF203067), BtCoV512 (NC_009657), 1A (NC_010437). Other coronavirus sequences used in this study: HCoV-229E
(AF304460), HCoV-OC43 (AY391777), HCoV-NL63 (AY567487), HKU1 (NC_006577), EMC (JX869059), FIPV (NC_002306), PRCV (DQ811787), BWCoV (NC_010646), MHV (AY700211), IBV (AY851295). AMPLIFICATION,
CLONING AND EXPRESSION OF THE BAT ACE2 GENE Construction of expression clones for human and civet ACE2 in pcDNA3.1 has been described previously29. Bat ACE2 was amplified from a _R.
sinicus_ (sample no. 3357). In brief, total RNA was extracted from bat rectal tissue using the RNeasy Mini Kit (Qiagen). First-strand complementary DNA was synthesized from total RNA by
reverse transcription with random hexamers. Full-length bat _ACE2_ fragments were amplified using forward primer bAF2 and reverse primer bAR2 (ref. 29). The _ACE2_ gene was cloned into
pCDNA3.1 with KpnI and XhoI, and verified by sequencing. Purified _ACE2_ plasmids were transfected to HeLa cells. After 24 h, lysates of HeLa cells expressing human, civet, or bat ACE2 were
confirmed by western blot or immunofluorescence assay. WESTERN BLOT ANALYSIS Lysates of cells or filtered supernatants containing pseudoviruses were separated by SDS–PAGE, followed by
transfer to a nitrocellulose membrane (Millipore). For detection of S protein, the membrane was incubated with rabbit anti-Rp3 S fragment (amino acids 561–666) polyantibodies (1:200), and
the bound antibodies were detected by alkaline phosphatase (AP)-conjugated goat anti-rabbit IgG (1:1,000). For detection of HIV-1 p24 in supernatants, monoclonal antibody against HIV p24
(p24 MAb) was used as the primary antibody at a dilution of 1:1,000, followed by incubation with AP-conjugated goat anti-mouse IgG at the same dilution. To detect the expression of ACE2 in
HeLa cells, goat antibody against the human ACE2 ectodomain (1:500) was used as the first antibody, followed by incubation with horseradish peroxidase-conjugated donkey anti-goat IgG
(1:1,000). VIRUS ISOLATION Vero E6 cell monolayers were maintained in DMEM supplemented with 10% FCS. PCR-positive samples (in 200 μl buffer) were gradient centrifuged at 3,000–12,000_g_,
and supernatant were diluted 1:10 in DMEM before being added to Vero E6 cells. After incubation at 37 °C for 1 h, inocula were removed and replaced with fresh DMEM with 2% FCS. Cells were
incubated at 37 °C for 3 days and checked daily for cytopathic effect. Double-dose triple antibiotics penicillin/streptomycin/amphotericin (Gibco) were included in all tissue culture media
(penicillin 200 IU ml−1, streptomycin 0.2 mg ml−1, amphotericin 0.5 μg ml−1). Three blind passages were carried out for each sample. After each passage, both the culture supernatant and cell
pellet were examined for presence of virus by RT–PCR using primers targeting the RdRP or S gene. Virions in supernatant (10 ml) were collected and fixed using 0.1% formaldehyde for 4 h,
then concentrated by ultracentrifugation through a 20% sucrose cushion (5 ml) at 80,000_g_ for 90 min using a Ty90 rotor (Beckman). The pelleted viral particles were suspended in 100 μl PBS,
stained with 2% phosphotungstic acid (pH 7.0) and examined using a Tecnai transmission electron microscope (FEI) at 200 kV. VIRUS INFECTIVITY DETECTED BY IMMUNOFLUORESCENCE ASSAY Cell lines
used for this study and their culture conditions are summarized in Extended Data Table 5. Virus titre was determined in Vero E6 cells by cytopathic effect (CPE) counts. Cell lines from
different origins and HeLa cells expressing ACE2 from human, civet or Chinese horseshoe bat were grown on coverslips in 24-well plates (Corning) incubated with bat SL-CoV-WIV1 at a
multiplicity of infection = 10 for 1 h. The inoculum was removed and washed twice with PBS and supplemented with medium. HeLa cells without ACE2 expression and Vero E6 cells were used as
negative and positive controls, respectively. At 24 h after infection, cells were washed with PBS and fixed with 4% formaldehyde in PBS (pH 7.4) for 20 min at 4 °C. ACE2 expression was
detected using goat anti-human ACE2 immunoglobulin (R&D Systems) followed by FITC-labelled donkey anti-goat immunoglobulin (PTGLab). Virus replication was detected using rabbit antibody
against the SL-CoV Rp3 nucleocapsid protein followed by Cy3-conjugated mouse anti-rabbit IgG. Nuclei were stained with DAPI. Staining patterns were examined using a FV1200 confocal
microscope (Olympus). VIRUS INFECTIVITY DETECTED BY REAL-TIME RT–PCR Vero E6, A549, PK15, RSKT and HeLa cells with or without expression of ACE2 of different origins were inoculated with 0.1
TCID50 WIV-1 and incubated for 1 h at 37 °C. After removing the inoculum, the cells were cultured with medium containing 1% FBS. Supernatants were collected at 0, 12, 24 and 48 h. RNA from
140 μl of each supernatant was extracted with the Viral RNA Mini Kit (Qiagen) following manufacturer’s instructions and eluted in 60 μl buffer AVE (Qiagen). RNA was quantified on the ABI
StepOne system, with the TaqMan AgPath-ID One-Step RT–PCR Kit (Applied Biosystems) in a 25 μl reaction mix containing 4 μl RNA, 1 × RT–PCR enzyme mix, 1 × RT–PCR buffer, 40 pmol forward
primer (5′-GTGGTGGTGACGGCAAAATG-3′), 40 pmol reverse primer (5′-AAGTGAAGCTTCTGGGCCAG-3′) and 12 pmol probe (5′-FAM-AAAGAGCTCAGCCCCAGATG-BHQ1-3′). Amplification parameters were 10 min at 50
°C, 10 min at 95 °C and 50 cycles of 15 s at 95 °C and 20 s at 60 °C. RNA dilutions from purified WIV-1 stock were used as a standard. SERUM NEUTRALIZATION TEST SARS patient sera were
inactivated at 56 °C for 30 min and then used for virus neutralization testing. Sera were diluted starting with 1:10 and then serially twofold diluted in 96-well cell plates to 1:40. Each
100 μl serum dilution was mixed with 100 μl viral supernatant containing 100 TCID50of WIV1 and incubated at 37 °C for 1 h. The mixture was added in triplicate wells of 96-well cell plates
with plated monolayers of Vero E6 cells and further incubated at 37 °C for 2 days. Serum from a healthy blood donor was used as a negative control in each experiment. CPE was observed using
an inverted microscope 2 days after inoculation. The neutralizing antibody titre was read as the highest dilution of serum which completely suppressed CPE in infected wells. The
neutralization test was repeated twice. RECOMBINATION ANALYSIS Full-length genomic sequences of SL-CoV Rs3367 or RsSHC014 were aligned with those of selected SARS-CoVs and bat SL-CoVs using
Clustal X. The aligned sequences were preliminarily scanned for recombination events using Recombination Detection Program (RDP) 4.0 (ref. 19). The potential recombination events suggested
by RDP owing to their strong _P_ values (<10–20) were investigated further by similarity plot and bootscan analyses implemented in Simplot 3.5.1. Phylogenetic origin of the major and
minor parental regions of Rs3367 or RsSHC014 were constructed from the concatenated sequences of the essential ORFs of the major and minor parental regions of selected SARS-CoV and SL-CoVs.
Two genome regions between three estimated breakpoints (20,827–26,553 and 26,554–28,685) were aligned independently using ClustalX and generated two alignments of 5,727 base pairs and 2,133
base pairs. The two alignments were used to construct maximum likelihood trees to better infer the fragment parents. All nucleotide numberings in this study are based on Rs3367 genome
position. ACCESSION CODES ACCESSIONS GENBANK/EMBL/DDBJ * KC880984 * KC881003 * KC881004 * KC881005 * KC881007 DATA DEPOSITS Sequences of three bat SL-CoV genomes, bat SL-CoV RBD and _R.
sinicus_ _ACE2_ genes have been deposited in GenBank under accession numbers KC881005–KC881007 (genomes from SL-CoV RsSHC014, Rs3367 and W1V1, respectively), KC880984–KC881003 (bat SL-CoV
RBD genes) and KC881004 (_R. sinicus ACE2_), respectively. REFERENCES * Ksiazek, T. G. et al. A novel coronavirus associated with severe acute respiratory syndrome. _N. Engl. J. Med._ 348,
1953–1966 (2003) Article CAS Google Scholar * Zaki, A. M., van Boheemen, S., Bestebroer, T. M., Osterhaus, A. D. & Fouchier, R. A. Isolation of a novel coronavirus from a man with
pneumonia in Saudi Arabia. _N. Engl. J. Med._ 367, 1814–1820 (2012) Article CAS Google Scholar * Anthony, S. J. et al. Coronaviruses in bats from Mexico. _J. Gen. Virol._ 94, 1028–1038
(2013) Article CAS Google Scholar * Raj, V. S. et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. _Nature_ 495, 251–254 (2013) Article ADS
CAS Google Scholar * Li, W. et al. Bats are natural reservoirs of SARS-like coronaviruses. _Science_ 310, 676–679 (2005) Article ADS CAS Google Scholar * Drexler, J. F. et al. Genomic
characterization of severe acute respiratory syndrome-related coronavirus in European bats and classification of coronaviruses based on partial RNA-dependent RNA polymerase gene sequences.
_J. Virol._ 84, 11336–11349 (2010) Article CAS Google Scholar * Tong, S. et al. Detection of novel SARS-like and other coronaviruses in bats from Kenya. _Emerg. Infect. Dis._ 15, 482–485
(2009) Article Google Scholar * Lau, S. K. P. et al. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. _Proc. Natl Acad. Sci. USA_ 102, 14040–14045 (2005)
Article ADS CAS Google Scholar * Ren, W. et al. Difference in receptor usage between severe acute respiratory syndrome (SARS) coronavirus and SARS-like coronavirus of bat origin. _J.
Virol._ 82, 1899–1907 (2008) Article CAS Google Scholar * Hon, C. C. et al. Evidence of the recombinant origin of a bat severe acute respiratory syndrome (SARS)-like coronavirus and its
implications on the direct ancestor of SARS coronavirus. _J. Virol._ 82, 1819–1826 (2008) Article CAS Google Scholar * Li, W. et al. Angiotensin-converting enzyme 2 is a functional
receptor for the SARS coronavirus. _Nature_ 426, 450–454 (2003) Article ADS CAS Google Scholar * Wong, S. K., Li, W., Moore, M. J., Choe, H. & Farzan, M. A 193-amino acid fragment of
the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. _J. Biol. Chem._ 279, 3197–3201 (2004) Article CAS Google Scholar * Becker, M. M. et al. Synthetic
recombinant bat SARS-like coronavirus is infectious in cultured cells and in mice. _Proc. Natl Acad. Sci. USA_ 105, 19944–19949 (2008) Article ADS CAS Google Scholar * Li, Y. et al. Host
range, prevalence, and genetic diversity of adenoviruses in bats. _J. Virol._ 84, 3889–3897 (2010) Article CAS Google Scholar * De Souza Luna, L. K. et al. Generic detection of
coronaviruses and differentiation at the prototype strain level by reverse transcription-PCR and nonfluorescent low-density microarray. _J. Clin. Microbiol._ 45, 1049–1052 (2007) Article
Google Scholar * Cui, J. et al. Evolutionary relationships between bat coronaviruses and their hosts. _Emerg. Infect. Dis._ 13, 1526–1532 (2007) Article CAS Google Scholar * Yuan, J. et
al. Intraspecies diversity of SARS-like coronaviruses in _Rhinolophus sinicus_ and its implications for the origin of SARS coronaviruses in humans. _J. Gen. Virol._ 91, 1058–1062 (2010)
Article CAS Google Scholar * Ren, W. et al. Full-length genome sequences of two SARS-like coronaviruses in horseshoe bats and genetic variation analysis. _J. Gen. Virol._ 87, 3355–3359
(2006) Article ADS CAS Google Scholar * Martin, D. P. et al. RDP3: a flexible and fast computer program for analyzing recombination. _Bioinformatics_ 26, 2462–2463 (2010) Article CAS
Google Scholar * Wu, K., Peng, G., Wilken, M., Geraghty, R. J. & Li, F. Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus. _J. Biol. Chem._ 287,
8904–8911 (2012) Article CAS Google Scholar * Li, W. et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. _EMBO J._ 24, 1634–1643 (2005) Article CAS
Google Scholar * Lau, S. K. et al. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related _Rhinolophus_ bat coronavirus in China
reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. _J. Virol._ 84, 2808–2819 (2010) Article CAS Google Scholar * Lau, S. K. et al. Coexistence
of different genotypes in the same bat and serological characterization of _Rousettus_ bat coronavirus HKU9 belonging to a novel Betacoronavirus subgroup. _J. Virol._ 84, 11385–11394 (2010)
Article CAS Google Scholar * Song, H. D. et al. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. _Proc. Natl Acad. Sci. USA_ 102, 2430–2435
(2005) Article ADS CAS Google Scholar * Memish, Z. A. et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. _Emerg. Infect. Dis._ 19, 11 (2013) Article Google
Scholar * Chan, J. F. et al. Is the discovery of the novel human betacoronavirus 2c EMC/2012 (HCoV-EMC) the beginning of another SARS-like pandemic? _J. Infect._ 65, 477–489 (2012) Article
Google Scholar * Ithete, N. L. et al. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. _Emerg. Infect. Dis._ 19, 1697–1699 (2013) Article Google
Scholar * Morse, S. S. et al. Prediction and prevention of the next pandemic zoonosis. _Lancet_ 380, 1956–1965 (2012) Article Google Scholar * Hou, Y. et al. Angiotensin-converting
enzyme 2 (ACE2) proteins of different bat species confer variable susceptibility to SARS-CoV entry. _Arch. Virol._ 155, 1563–1569 (2010) Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS We acknowledge financial support from the State Key Program for Basic Research (2011CB504701 and 2010CB530100), National Natural Science Foundation of China (81290341 and
31321001), Scientific and technological basis special project (2013FY113500), CSIRO OCE Science Leaders Award, National Institute of Allergy and Infectious Diseases (NIAID) award number
R01AI079231, a National Institutes of Health (NIH)/National Science Foundation (NSF) ‘Ecology and Evolution of Infectious Diseases’ award from the NIH Fogarty International Center
(R01TW005869), an award from the NIH Fogarty International Center supported by International Influenza Funds from the Office of the Secretary of the Department of Health and Human Services
(R56TW009502), and United States Agency for International Development (USAID) Emerging Pandemic Threats PREDICT. The contents are the responsibility of the authors and do not necessarily
reflect the views of NIAID, NIH, NSF, USAID or the United States Government. We thank X. Che from Zhujiang Hospital, Southern Medical University, for providing human SARS patient sera.
AUTHOR INFORMATION Author notes * Xing-Yi Ge, Jia-Lu Li and Xing-Lou Yang: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Center for Emerging Infectious Diseases,
State Key Laboratory of Virology, Wuhan Institute of Virology of the Chinese Academy of Sciences, Wuhan, 430071, China Xing-Yi Ge, Jia-Lu Li, Xing-Lou Yang, Ben Hu, Wei Zhang, Cheng Peng,
Yu-Ji Zhang, Chu-Ming Luo, Bing Tan, Ning Wang, Yan Zhu & Zheng-Li Shi * EcoHealth Alliance, New York, 10001, New York, USA Aleksei A. Chmura, Guangjian Zhu, Jonathan H. Epstein &
Peter Daszak * One Health Institute, School of Veterinary Medicine, University of California, Davis, 95616, California, USA Jonna K. Mazet * CSIRO Australian Animal Health Laboratory,
Geelong, 3220, Victoria, Australia Gary Crameri & Lin-Fa Wang * College of Life Sciences, East China Normal University, Shanghai 200062, China, Shu-Yi Zhang * Emerging Infectious
Diseases Program, Duke-NUS Graduate Medical School, Singapore 169857, Lin-Fa Wang Authors * Xing-Yi Ge View author publications You can also search for this author inPubMed Google Scholar *
Jia-Lu Li View author publications You can also search for this author inPubMed Google Scholar * Xing-Lou Yang View author publications You can also search for this author inPubMed Google
Scholar * Aleksei A. Chmura View author publications You can also search for this author inPubMed Google Scholar * Guangjian Zhu View author publications You can also search for this author
inPubMed Google Scholar * Jonathan H. Epstein View author publications You can also search for this author inPubMed Google Scholar * Jonna K. Mazet View author publications You can also
search for this author inPubMed Google Scholar * Ben Hu View author publications You can also search for this author inPubMed Google Scholar * Wei Zhang View author publications You can also
search for this author inPubMed Google Scholar * Cheng Peng View author publications You can also search for this author inPubMed Google Scholar * Yu-Ji Zhang View author publications You
can also search for this author inPubMed Google Scholar * Chu-Ming Luo View author publications You can also search for this author inPubMed Google Scholar * Bing Tan View author
publications You can also search for this author inPubMed Google Scholar * Ning Wang View author publications You can also search for this author inPubMed Google Scholar * Yan Zhu View
author publications You can also search for this author inPubMed Google Scholar * Gary Crameri View author publications You can also search for this author inPubMed Google Scholar * Shu-Yi
Zhang View author publications You can also search for this author inPubMed Google Scholar * Lin-Fa Wang View author publications You can also search for this author inPubMed Google Scholar
* Peter Daszak View author publications You can also search for this author inPubMed Google Scholar * Zheng-Li Shi View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS Z.-L.S. and P.D. designed and coordinated the study. X.-Y.G., J.-L. L. and X.-L.Y. conducted majority of experiments and contributed equally to the study.
A.A.C., B.H., W.Z., C.P., Y.-J.Z., C.-M.L., B.T., N.W. and Y.Z. conducted parts of the experiments and analyses. J.H.E., J.K.M. and S.-Y.Z. coordinated the field study. X.-Y.G., J.-L.L.,
X.-L.Y., B.T. and G.-J.Z. collected the samples. G.C. and L.-F.W. designed and supervised part of the experiments. All authors contributed to the interpretations and conclusions presented.
Z.-L.S. and X-Y.G. wrote the manuscript with significant contributions from P.D. and L-F.W. and input from all authors. CORRESPONDING AUTHORS Correspondence to Peter Daszak or Zheng-Li Shi.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIGURE 1 SEQUENCE ALIGNMENT OF COV S PROTEIN RBD.
SARS-CoV S protein (amino acids 310–520) is aligned with homologous regions of bat SL-CoVs using ClustalW. The newly discovered bat SL-CoVs are indicated with a bold vertical line on the
left. The key amino acid residues involved in the interaction with human ACE2 are numbered on the top of the aligned sequences. EXTENDED DATA FIGURE 2 ALIGNMENT OF COV S PROTEIN S1
SEQUENCES. Alignment of S1 sequences (amino acids 1–660) of the two novel bat SL-CoV S proteins with those of previously reported bat SL-CoVs and human and civet SARS-CoVs. The newly
discovered bat SL-CoVs are boxed in red. SARS-CoV GZ02, BJ01 and Tor2 were isolated from patients in the early, middle and late phase, respectively, of the SARS outbreak in 2003. SARS-CoV
SZ3 was identified from _P. larvata_ in 2003 collected in Guangdong, China. SL-CoV Rp3, Rs 672 and HKU3-1 were identified from _R. sinicus_ collected in Guangxi, Guizhou and Hong Kong,
China, respectively. Rf1 and Rm1 were identified from _R. ferrumequinum_ and _R. macrotis_, respectively, collected in Hubei Province, China. Bat SARS-related CoV BM48-31 was identified from
_R. blasii_ collected in Bulgaria. EXTENDED DATA FIGURE 3 COMPLETE RDRP SEQUENCE PHYLOGENY. Phylogenetic tree of bat SL-CoVs and SARS-CoVs on the basis of complete RdRP sequences (2,796
nucleotides). Bat SL-CoVs RsSHC014 and Rs3367 are highlighted by filled circles. Three established coronaivirus genera, _Alphacoronavirus_, _Betacoronavirus_ and _Gammacoronavirus_ are
marked as α, β and γ, respectively. Four CoV groups in the genus _Betacoronavirus_ are indicated as A, B, C and D, respectively. MHV, murine hepatitis virus; PHEV, porcine haemagglutinating
encephalomyelitis virus; PRCV, porcine respiratory coronavirus; FIPV, feline infectious peritonitis virus; IBV, infectious bronchitis coronavirus; BW, beluga whale coronavirus. EXTENDED DATA
FIGURE 4 SEQUENCE PHYLOGENY OF THE COMPLETE S PROTEIN OF SL-COVS AND SARS-COV. Phylogenetic tree of bat SL-CoVs and SARS-CoVs on the basis of complete S protein sequences (1,256 amino
acids). Bat SL-CoVs RsSHC014 and Rs3367 are highlighted by filled circles. Bat CoV HKU9 was used as an outgroup. EXTENDED DATA FIGURE 5 DETECTION OF POTENTIAL RECOMBINATION EVENTS. A, B,
Similarity plot (A) and bootscan analysis (B) detected three recombination breakpoints in the bat SL-CoV Rs3367 or SHC014 genome. The three breakpoints were located at the ORF1b (nt 20,827),
M (nucleotides 26,553) and N (nucleotides 28,685) genes, respectively. Both analyses were performed with an F84 distance model, a window size of 1,500 base pairs and a step size of 300 base
pairs. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Ge, XY., Li, JL., Yang, XL. _et al._ Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. _Nature_ 503, 535–538
(2013). https://doi.org/10.1038/nature12711 Download citation * Received: 16 May 2013 * Accepted: 18 September 2013 * Published: 30 October 2013 * Issue Date: 28 November 2013 * DOI:
https://doi.org/10.1038/nature12711 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
