Structure of the secy channel during initiation of protein translocation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

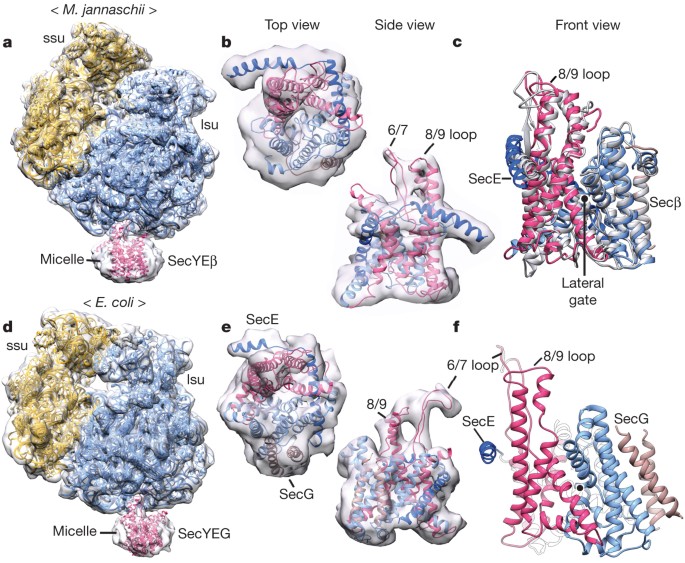
ABSTRACT Many secretory proteins are targeted by signal sequences to a protein-conducting channel, formed by prokaryotic SecY or eukaryotic Sec61 complexes, and are translocated across the
membrane during their synthesis1,2. Crystal structures of the inactive channel show that the SecY subunit of the heterotrimeric complex consists of two halves that form an hourglass-shaped
pore with a constriction in the middle of the membrane and a lateral gate that faces the lipid phase3,4,5. The closed channel has an empty cytoplasmic funnel and an extracellular funnel that
is filled with a small helical domain, called the plug. During initiation of translocation, a ribosome–nascent chain complex binds to the SecY (or Sec61) complex, resulting in insertion of
the nascent chain. However, the mechanism of channel opening during translocation is unclear. Here we have addressed this question by determining structures of inactive and active
ribosome–channel complexes with cryo-electron microscopy. Non-translating ribosome–SecY channel complexes derived from _Methanocaldococcus jannaschii_ or _Escherichia coli_ show the channel
in its closed state, and indicate that ribosome binding per se causes only minor changes. The structure of an active _E. coli_ ribosome–channel complex demonstrates that the nascent chain
opens the channel, causing mostly rigid body movements of the amino- and carboxy-terminal halves of SecY. In this early translocation intermediate, the polypeptide inserts as a loop into the
SecY channel with the hydrophobic signal sequence intercalated into the open lateral gate. The nascent chain also forms a loop on the cytoplasmic surface of SecY rather than entering the
channel directly. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution
Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full
article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs *
Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS STEPWISE GATING OF THE SEC61 PROTEIN-CONDUCTING CHANNEL BY SEC63 AND SEC62 Article 04 January 2021 VISUALIZATION OF
TRANSLATION AND PROTEIN BIOGENESIS AT THE ER MEMBRANE Article Open access 25 January 2023 RIBOSOME PROFILING REVEALS MULTIPLE ROLES OF SECA IN COTRANSLATIONAL PROTEIN EXPORT Article Open
access 13 June 2022 ACCESSION CODES PRIMARY ACCESSIONS ELECTRON MICROSCOPY DATA BANK * 5691 * 5692 * 5693 PROTEIN DATA BANK * 1VVK * 3J43 * 3J44 * 3J45 * 3J46 DATA DEPOSITS Electron density
maps have been submitted to the Electron Microscopy Data Bank (http://www.emdatabank.org/) under accession numbers EMD-5691, EMD-5692 and EMD-5693, and modelled structures to the Protein
Data Bank (http://www.rcsb.org/pdb/home/home.do) under accession numbers 3J43, 3J44, 3J45, 3J46 and 1VVK. REFERENCES * Park, E. & Rapoport, T. A. Mechanisms of Sec61/SecY-mediated
protein translocation across membranes. _Annu. Rev. Biophys._ 41, 21–40 (2012) Article CAS Google Scholar * Shao, S. & Hegde, R. S. Membrane protein insertion at the endoplasmic
reticulum. _Annu. Rev. Cell Dev. Biol._ 27, 25–56 (2011) Article CAS Google Scholar * Van den Berg, B. et al. X-ray structure of a protein-conducting channel. _Nature_ 427, 36–44 (2004)
Article CAS Google Scholar * Tsukazaki, T. et al. Conformational transition of Sec machinery inferred from bacterial SecYE structures. _Nature_ 455, 988–991 (2008) Article ADS CAS
Google Scholar * Egea, P. F. & Stroud, R. M. Lateral opening of a translocon upon entry of protein suggests the mechanism of insertion into membranes. _Proc. Natl Acad. Sci. USA_ 107,
17182–17187 (2010) Article ADS CAS Google Scholar * Armache, J. P. et al. Promiscuous behaviour of archaeal ribosomal proteins: implications for eukaryotic ribosome evolution. _Nucleic
Acids Res._ 41, 1284–1293 (2013) Article CAS Google Scholar * Trabuco, L. G., Villa, E., Mitra, K., Frank, J. & Schulten, K. Flexible fitting of atomic structures into electron
microscopy maps using molecular dynamics. _Structure_ 16, 673–683 (2008) Article CAS Google Scholar * Ménétret, J. F. et al. Ribosome binding of a single copy of the SecY complex:
implications for protein translocation. _Mol. Cell_ 28, 1083–1092 (2007) Article Google Scholar * Ménétret, J. F. et al. Single copies of Sec61 and TRAP associate with a nontranslating
mammalian ribosome. _Structure_ 16, 1126–1137 (2008) Article Google Scholar * Becker, T. et al. Structure of monomeric yeast and mammalian Sec61 complexes interacting with the translating
ribosome. _Science_ 326, 1369–1373 (2009) Article ADS CAS Google Scholar * Frauenfeld, J. et al. Cryo-EM structure of the ribosome–SecYE complex in the membrane environment. _Nature
Struct. Mol. Biol._ 18, 614–621 (2011) Article CAS Google Scholar * Berk, V., Zhang, W., Pai, R. D. & Cate, J. H. D. Structural basis for mRNA and tRNA positioning on the ribosome.
_Proc. Natl Acad. Sci. USA_ 103, 15830–15834 (2006) Article ADS CAS Google Scholar * Zimmer, J., Nam, Y. & Rapoport, T. A. Structure of a complex of the ATPase SecA and the
protein-translocation channel. _Nature_ 455, 936–943 (2008) Article ADS CAS Google Scholar * Park, E. & Rapoport, T. A. Preserving the membrane barrier for small molecules during
bacterial protein translocation. _Nature_ 473, 239–242 (2011) Article ADS CAS Google Scholar * Park, E. & Rapoport, T. A. Bacterial protein translocation requires only one copy of
the SecY complex _in vivo_ . _J. Cell Biol._ 198, 881–893 (2012) Article CAS Google Scholar * Schierle, C. F. et al. The DsbA signal sequence directs efficient, cotranslational export of
passenger proteins to the _Escherichia coli_ periplasm via the signal recognition particle pathway. _J. Bacteriol._ 185, 5706–5713 (2003) Article CAS Google Scholar * Nakatogawa, H. &
Ito, K. The ribosomal exit tunnel functions as a discriminating gate. _Cell_ 108, 629–636 (2002) Article CAS Google Scholar * Zhang, Y. et al. MazF cleaves cellular mRNAs specifically at
ACA to block protein synthesis in _Escherichia coli_ . _Mol. Cell_ 12, 913–923 (2003) Article CAS Google Scholar * Muto, H., Nakatogawa, H. & Ito, K. Genetically encoded but
nonpolypeptide prolyl-tRNA functions in the A site for SecM-mediated ribosomal stall. _Mol. Cell_ 22, 545–552 (2006) Article CAS Google Scholar * Sengupta, J., Agrawal, R. K. & Frank,
J. Visualization of protein S1 within the 30S ribosomal subunit and its interaction with messenger RNA. _Proc. Natl Acad. Sci. USA_ 98, 11991–11996 (2001) Article ADS CAS Google Scholar
* Lycklama a Nijeholt, J. A., Bulacu, M., Marrink, S. J. & Driessen, A. J. Immobilization of the plug domain inside the SecY channel allows unrestricted protein translocation. _J.
Biol. Chem._ 285, 23747–23754 (2010) Article CAS Google Scholar * Plath, K., Mothes, W., Wilkinson, B. M., Stirling, C. J. & Rapoport, T. A. Signal sequence recognition in
posttranslational protein transport across the yeast ER membrane. _Cell_ 94, 795–807 (1998) Article CAS Google Scholar * Knyazev, D. G. et al. The bacterial translocon SecYEG opens upon
ribosome binding. _J. Biol. Chem._ 288, 17941–17946 (2013) Article CAS Google Scholar * Shaw, A. S., Rottier, P. J. & Rose, J. K. Evidence for the loop model of signal-sequence
insertion into the endoplasmic reticulum. _Proc. Natl Acad. Sci. USA_ 85, 7592–7596 (1988) Article ADS CAS Google Scholar * Hizlan, D. et al. Structure of the SecY complex unlocked by a
preprotein mimic. _Cell Rep_ 1, 21–28 (2012) Article CAS Google Scholar * Matlack, K. E., Misselwitz, B., Plath, K. & Rapoport, T. A. BiP acts as a molecular ratchet during
posttranslational transport of prepro-α factor across the ER membrane. _Cell_ 97, 553–564 (1999) Article CAS Google Scholar * Nicchitta, C. V. & Blobel, G. Lumenal proteins of the
mammalian endoplasmic reticulum are required to complete protein translocation. _Cell_ 73, 989–998 (1993) Article CAS Google Scholar * Tsukazaki, T. et al. Structure and function of a
membrane component SecDF that enhances protein export. _Nature_ 474, 235–238 (2011) Article CAS Google Scholar * Tang, G. et al. EMAN2: an extensible image processing suite for electron
microscopy. _J. Struct. Biol._ 157, 38–46 (2007) Article CAS Google Scholar * Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. _J.
Comput. Chem._ 25, 1605–1612 (2004) Article CAS Google Scholar * Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in _Escherichia coli_ K-12 using PCR
products. _Proc. Natl Acad. Sci. USA_ 97, 6640–6645 (2000) Article ADS CAS Google Scholar * Alami, M., Dalal, K., Lelj-Garolla, B., Sligar, S. G. & Duong, F. Nanodiscs unravel the
interaction between the SecYEG channel and its cytosolic partner SecA. _EMBO J._ 26, 1995–2004 (2007) Article CAS Google Scholar * Nath, A., Atkins, W. M. & Sligar, S. G. Applications
of phospholipid bilayer nanodiscs in the study of membranes and membrane proteins. _Biochemistry_ 46, 2059–2069 (2007) Article CAS Google Scholar * Cannon, K. S., Or, E., Clemons, W. M.,
Jr, Shibata, Y. & Rapoport, T. A. Disulfide bridge formation between SecY and a translocating polypeptide localizes the translocation pore to the center of SecY. _J. Cell Biol._ 169,
219–225 (2005) Article CAS Google Scholar * Ludtke, S. J., Baldwin, P. R. & Chiu, W. EMAN: semiautomated software for high-resolution single-particle reconstructions. _J. Struct.
Biol._ 128, 82–97 (1999) Article CAS Google Scholar * Jenner, L. B., Demeshkina, N., Yusupova, G. & Yusupov, M. Structural aspects of messenger RNA reading frame maintenance by the
ribosome. _Nature Struct. Mol. Biol._ 17, 555–560 (2010) Article CAS Google Scholar * Emsley, P. & Cowtan, K. _Coot_: model-building tools for molecular graphics. _Acta Crystallogr.
D_ 60, 2126–2132 (2004) Article Google Scholar * Phillips, J. C. et al. Scalable molecular dynamics with NAMD. _J. Comput. Chem._ 26, 1781–1802 (2005) Article CAS Google Scholar *
DiMaio, F., Tyka, M. D., Baker, M. L., Chiu, W. & Baker, D. Refinement of protein structures into low-resolution density maps using Rosetta. _J. Mol. Biol._ 392, 181–190 (2009) Article
CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank K. Matlack and T. Guettler for reading the manuscript. This work was supported by National Institutes of Health grants
GM067887 to J.C.G., GM080139 to S.J.L., GM052586 to T.A.R. and GM45377 to C.W.A. T.A.R. is a Howard Hughes Institute investigator. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Cell Biology and Howard Hughes Medical Institute, Harvard Medical School, 240 Longwood Avenue, Boston, Massachusetts 02115, USA, Eunyong Park, Weikai Li, Andrew Whynot & Tom A. Rapoport
* Department of Physiology and Biophysics, Boston University School of Medicine, 700 Albany Street, Boston, Massachusetts 02118-2526, USA, Jean-François Ménétret & Christopher W. Akey *
School of Physics, Georgia Institute of Technology, Atlanta, 30332, Georgia, USA James C. Gumbart * Verna and Marrs McLean Department of Biochemistry and Molecular Biology, National Center
for Macromolecular Imaging, Baylor College of Medicine, 1 Baylor Plaza, Houston, Texas 77030, USA, Steven J. Ludtke Authors * Eunyong Park View author publications You can also search for
this author inPubMed Google Scholar * Jean-François Ménétret View author publications You can also search for this author inPubMed Google Scholar * James C. Gumbart View author publications
You can also search for this author inPubMed Google Scholar * Steven J. Ludtke View author publications You can also search for this author inPubMed Google Scholar * Weikai Li View author
publications You can also search for this author inPubMed Google Scholar * Andrew Whynot View author publications You can also search for this author inPubMed Google Scholar * Tom A.
Rapoport View author publications You can also search for this author inPubMed Google Scholar * Christopher W. Akey View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS E.P. designed and purified RNC–channel complexes, J.-F.M. and C.W.A. obtained and analysed the electron microscopy data, J.C.G. helped with MDFF and channel
modelling, S.J.L. helped with data analysis, W.L. and A.W. purified _M. jannaschii_ components, and T.A.R., E.P. and C.W.A. wrote the paper. CORRESPONDING AUTHORS Correspondence to Tom A.
Rapoport or Christopher W. Akey. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION This file
contains Supplementary Figures 1-22 and Supplementary Tables 1-3. (PDF 15975 kb) CONFORMATIONAL CHANGES IN THE E. COLI SECY CHANNEL DURING TRANSLOCATION - PART I The video illustrates
conformational changes in the SecY channel that occur when a nascent chain with a signal sequence is bound in a looped configuration. The nascent chain has been omitted for clarity. A morph
was generated between models of the closed and active SecY complex in Chimera, with the channel viewed from the front. The N-terminal half of SecY is in light blue, the C-terminal half is in
red, SecE is dark blue, SecG is brown, and the plug is yellow. Six isoleucines that line the pore are shown as ball and stick representations in dark grey. Ribosomal helices H6, H7, H50 and
H50 are shown in white; proteins L23, and L24 are green, and L29 is dark grey. The position and conformation of the 6/7 loop was fixed during the simulation. Note that SecE tilts to
accommodate movements of the two halves of SecY, and helix 8b moves downwards towards the lateral gate, while helix 9 remains anchored to the large ribosomal subunit. These movements open
the lateral gate between the two halves of SecY. (MOV 5307 kb) CONFORMATIONAL CHANGES IN THE E. COLI SECY CHANNEL DURING TRANSLOCATION - PART II The video illustrates conformational changes
in SecY channel that occur when a nascent chain with a signal sequence is bound in a looped configuration. The channel is viewed from the top. The nascent chain has been omitted for clarity.
Note that the N-terminal half of SecY rotates and tilts backwards, approximating a rigid body movement that also involves SecG. However, helix 3 bends and the junction between helix 5 and 6
also moves to accommodate these large movements. These movements create a large translocation pore with access to the opened lateral gate. (MOV 5618 kb) VISUALIZING THE NASCENT CHAIN IN THE
ACTIVE E. COLI SECY CHANNEL The video illustrates the orientation of a closed SecY channel beneath the ribosome and then shows a progression from a closed to an open SecY. The nascent chain
containing the signal sequence helix (residues 1-73; in green) is bound in a looped configuration and a cytoplasmic loop resides in a V-shaped canyon bounded by the 6/7 connection and helix
10. The N-terminal half of SecY is in light blue, the C-terminal half is in red, SecE is dark blue, SecG is brown, and the plug is yellow. Six isoleucines that line the pore are shown as
ball and stick representations in dark grey. Ribosomal helices H6, H7, H50 and H59 are shown in white; ribosomal proteins are colored as follows: L23 (purple), L29 (dark grey) and L29
(yellow). The extended nascent chain exiting the tunnel couldinteract with the 6/7 loop to help stabilize the translocating ribosomechannel complex. (MOV 12322 kb) POWERPOINT SLIDES
POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Park, E., Ménétret, JF., Gumbart, J. _et al._ Structure of the SecY channel during initiation of protein translocation. _Nature_ 506, 102–106 (2014).
https://doi.org/10.1038/nature12720 Download citation * Received: 11 June 2013 * Accepted: 04 October 2013 * Published: 23 October 2013 * Issue Date: 06 February 2014 * DOI:
https://doi.org/10.1038/nature12720 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
