Inhibition of cell expansion by rapid abp1-mediated auxin effect on microtubules
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The prominent and evolutionarily ancient role of the plant hormone auxin is the regulation of cell expansion1. Cell expansion requires ordered arrangement of the cytoskeleton2 but
molecular mechanisms underlying its regulation by signalling molecules including auxin are unknown. Here we show in the model plant _Arabidopsis thaliana_ that in elongating cells exogenous
application of auxin or redistribution of endogenous auxin induces very rapid microtubule re-orientation from transverse to longitudinal, coherent with the inhibition of cell expansion. This
fast auxin effect requires auxin binding protein 1 (ABP1) and involves a contribution of downstream signalling components such as ROP6 GTPase, ROP-interactive protein RIC1 and the
microtubule-severing protein katanin. These components are required for rapid auxin- and ABP1-mediated re-orientation of microtubules to regulate cell elongation in roots and dark-grown
hypocotyls as well as asymmetric growth during gravitropic responses. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution
ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article *
Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn
about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ABP1–TMK AUXIN PERCEPTION FOR GLOBAL PHOSPHORYLATION AND AUXIN
CANALIZATION Article 07 September 2022 MECHANISMS OF AUXIN ACTION IN PLANT GROWTH AND DEVELOPMENT Article 19 May 2025 REGULATION OF AUXIN RESPONSE FACTOR CONDENSATION AND NUCLEO-CYTOPLASMIC
PARTITIONING Article Open access 11 July 2022 REFERENCES * Perrot-Rechenmann, C. Cellular responses to auxin: division versus expansion. _Cold Spring Harb. Perspect. Biol._ 2, a001446 (2010)
Article Google Scholar * Sedbrook, J. C. & Kaloriti, D. Microtubules, MAPs and plant directional cell expansion. _Trends Plant Sci._ 13, 303–310 (2008) Article CAS Google Scholar *
Chapman, E. J. & Estelle, M. Mechanism of auxin-regulated gene expression in plants. _Annu. Rev. Genet._ 43, 265–285 (2009) Article CAS Google Scholar * Lucas, J. & Shaw, S. L.
Cortical microtubule arrays in the _Arabidopsis_ seedling. _Curr. Opin. Plant Biol._ 11, 94–98 (2008) Article CAS Google Scholar * Blancaflor, E. B. The cytoskeleton and gravitropism in
higher plants. _J. Plant Growth Regul._ 21, 120–136 (2002) Article CAS Google Scholar * Lindeboom, J. J. et al. A mechanism for reorientation of cortical microtubule arrays driven by
microtubule severing. _Science_ 342, 1245533 (2013) Article Google Scholar * Ubeda-Tomas, S., Beemster, G. T. & Bennett, M. J. Hormonal regulation of root growth: integrating local
activities into global behaviour. _Trends Plant Sci._ 17, 326–331 (2012) Article CAS Google Scholar * Marc, J. et al. A GFP-MAP4 reporter gene for visualizing cortical microtubule
rearrangements in living epidermal cells. _Plant Cell_ 10, 1927–1940 (1998) CAS PubMed PubMed Central Google Scholar * Chan, J., Calder, G., Fox, S. & Lloyd, C. Cortical microtubule
arrays undergo rotary movements in _Arabidopsis_ hypocotyl epidermal cells. _Nature Cell Biol._ 9, 171–175 (2007) Article CAS Google Scholar * Collett, C. E., Harberd, N. P. & Leyser,
O. Hormonal interactions in the control of _Arabidopsis_ hypocotyl elongation. _Plant Physiol._ 124, 553–562 (2000) Article CAS Google Scholar * Simon, S. et al. Defining the selectivity
of processes along the auxin response chain: a study using auxin analogues. _New Phytol._ 200, 1034–1048 (2013) Article CAS Google Scholar * Morita, M. T. Directional gravity sensing in
gravitropism. _Annu. Rev. Plant Biol._ 61, 705–720 (2010) Article CAS Google Scholar * Chan, J., Calder, G. M., Doonan, J. H. & Lloyd, C. W. EB1 reveals mobile microtubule nucleation
sites in _Arabidopsis_. _Nature Cell Biol._ 5, 967–971 (2003) Article CAS Google Scholar * Brunoud, G. et al. A novel sensor to map auxin response and distribution at high spatio-temporal
resolution. _Nature_ 482, 103–106 (2012) Article ADS CAS Google Scholar * Tromas, A. et al. Auxin-binding protein 1 is a negative regulator of the SCFTIR1/AFB pathway. _Nature Commun._
4, 2496 (2013) Article ADS Google Scholar * Xu, T. et al. Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in _Arabidopsis_. _Cell_ 143, 99–110 (2010)
Article CAS Google Scholar * Robert, S. et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in _Arabidopsis_. _Cell_ 143, 111–121 (2010) Article CAS Google Scholar
* Dharmasiri, N. et al. Plant development is regulated by a family of auxin receptor F box proteins. _Dev. Cell_ 9, 109–119 (2005) Article CAS Google Scholar * Braun, N. et al.
Conditional repression of AUXIN BINDING PROTEIN1 reveals that it coordinates cell division and cell expansion during postembryonic shoot development in _Arabidopsis_ and tobacco. _Plant
Cell_ 20, 2746–2762 (2008) Article CAS Google Scholar * Tromas, A. et al. The AUXIN BINDING PROTEIN 1 is required for differential auxin responses mediating root growth. _PLoS ONE_ 4,
e6648 (2009) Article ADS Google Scholar * Hayashi, K. et al. Small-molecule agonists and antagonists of F-box protein-substrate interactions in auxin perception and signaling. _Proc. Natl
Acad. Sci. USA_ 105, 5632–5637 (2008) Article ADS CAS Google Scholar * Shishova, M. & Lindberg, S. A new perspective on auxin perception. _J. Plant Physiol._ 167, 417–422 (2010)
Article CAS Google Scholar * Uyttewaal, M. et al. Mechanical stress acts via katanin to amplify differences in growth rate between adjacent cells in _Arabidopsis_. _Cell_ 149, 439–451
(2012) Article CAS Google Scholar * Lin, D. et al. Rho GTPase signaling activates microtubule severing to promote microtubule ordering in _Arabidopsis_. _Curr. Biol._ 23, 290–297 (2013)
Article CAS Google Scholar * Chen, X. et al. ABP1 and ROP6 GTPase signaling regulate clathrin-mediated endocytosis in _Arabidopsis_ roots. _Curr. Biol._ 22, 1326–1332 (2012) Article CAS
Google Scholar * Paciorek, T. et al. Auxin inhibits endocytosis and promotes its own efflux from cells. _Nature_ 435, 1251–1256 (2005) Article ADS CAS Google Scholar * Montagnac, G.
et al. αTAT1 catalyses microtubule acetylation at clathrin-coated pits. _Nature_ 502, 567–570 (2013) Article ADS CAS Google Scholar * Boudaoud, A. et al. FibrilTool, an ImageJ plug-in to
quantify fibrillar structures in raw microscopy images. _Nature Protocols_ 9, 457–463 (2014) Article CAS Google Scholar * Salaycik, K. J., Fagerstrom, C. J., Murthy, K., Tulu, U. S.
& Wadsworth, P. Quantification of microtubule nucleation, growth and dynamics in wound-edge cells. _J. Cell Sci._ 118, 4113–4122 (2005) Article CAS Google Scholar * Leblanc, N. et al.
A novel immunological approach establishes that the auxin-binding protein, Nt-abp1, is an element involved in auxin signaling at the plasma membrane. _J. Biol. Chem._ 274, 28314–28320
(1999) Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank R. Dixit for performing complementary experiments, D. W. Ehrhardt and T. Hashimoto for providing the seeds
of TUB6–RFP and EB1b–GFP respectively, E. Zazimalova, J. Petrasek and M. Fendrych for discussing the manuscript and J. Leung for text optimization. This work was supported by the European
Research Council (project ERC-2011-StG-20101109-PSDP, to J.F.), ANR blanc AuxiWall project (ANR-11-BSV5-0007, to C.P.-R. and L.G.) and the Agency for Innovation by Science and Technology
(IWT) (to H.R.). This work benefited from the facilities and expertise of the Imagif Cell Biology platform (http://www.imagif.cnrs.fr), which is supported by the Conseil Général de
l’Essonne. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of Science and Technology Austria (IST Austria), Am Campus 1, 3400 Klosterneuburg, Austria, Xu Chen, Hongjiang Li, Robert
Hauschild, Hana Rakusová, Eva Benkova & Jiří Friml * Department of Plant Systems Biology, Vlaams Instituut voor Biotechnologie (VIB), Ghent University, B-9052 Gent, Belgium, Xu Chen,
Hongjiang Li, Anas Abuzeineh, Hana Rakusová, Eva Benkova & Jiří Friml * Department of Plant Biotechnology and Genetics, Ghent University, B-9052 Gent, Belgium, Xu Chen, Hongjiang Li,
Anas Abuzeineh, Hana Rakusová, Eva Benkova & Jiří Friml * Institut des Sciences du Végétal, UPR2355 CNRS, Saclay Plant Sciences LabEx, 1 Avenue de la Terrasse, 91198 Gif sur Yvette,
Cedex, France, Laurie Grandont, Sébastien Paque & Catherine Perrot-Rechenmann Authors * Xu Chen View author publications You can also search for this author inPubMed Google Scholar *
Laurie Grandont View author publications You can also search for this author inPubMed Google Scholar * Hongjiang Li View author publications You can also search for this author inPubMed
Google Scholar * Robert Hauschild View author publications You can also search for this author inPubMed Google Scholar * Sébastien Paque View author publications You can also search for this
author inPubMed Google Scholar * Anas Abuzeineh View author publications You can also search for this author inPubMed Google Scholar * Hana Rakusová View author publications You can also
search for this author inPubMed Google Scholar * Eva Benkova View author publications You can also search for this author inPubMed Google Scholar * Catherine Perrot-Rechenmann View author
publications You can also search for this author inPubMed Google Scholar * Jiří Friml View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS X.C.,
L.G., C.P.-R. and J.F. conceived the study and designed experiments. X.C. performed experiments in roots, and L.G. performed experiments in hypocotyls. H.L., S.P. and A.A. assisted in
microscopy and data generation. H.R. generated partial double mutants. R.H. did bioinformatics analysis. E.B. helped with discussion of the data. X.C., L.G., C.P.-R. and J.F. wrote the
manuscript. CORRESPONDING AUTHORS Correspondence to Catherine Perrot-Rechenmann or Jiří Friml. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests.
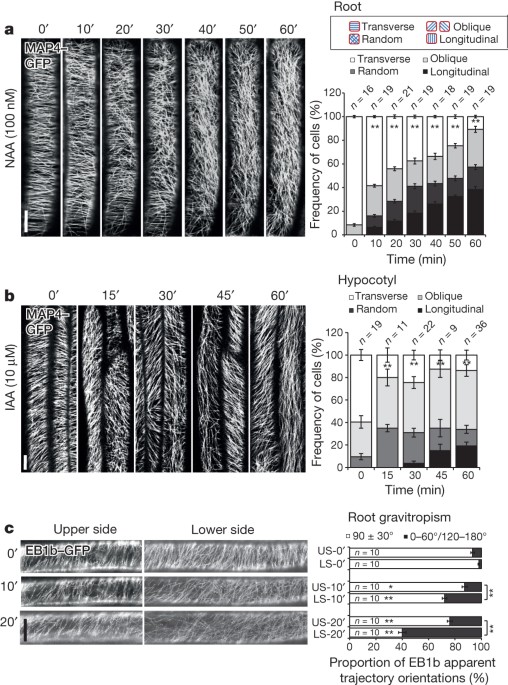
EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIGURE 1 AUXIN INDUCES MICROTUBULE REARRANGEMENT IN ROOT CELLS. A, Schematic diagram of root and dark-grown hypocotyl growth. The growth
direction of the root and hypocotyl is named as the cell growth axis. The observed cells for microtubules array were in the transition zone (highlighted by red line) of roots and in the
elongation zone of dark-grown hypocotyls (highlighted by grey frame). The arrays of microtubules in root and hypocotyl were depicted for the expanding cells. B–F, MAP4–GFP or TUA6–RFP
visualization of microtubule orientation in roots was performed by time-lapse observation (every 10 min; prime (′), minutes) following 100 nM NAA or IAA treatment, and deviated angles of
individual microtubules were quantified as transverse microtubules (90 ± 30°) or longitudinal microtubules (0–60°/120–180°). In C and F, Student’s _t_-test was calculated for transverse
microtubules compared with untreated roots (*_P_ < 0.05; **_P_ < 0.001). G, H, MAP4–GFP visualization and quantification of microtubule orientation in roots after 1 µM 2-NAA treatment
for 60 min or after transfer of seedlings on acidified 1/2 Murashige and Skoog medium at pH 4.9 for 30, 90 and 180 minutes. Student’s _t_-test was calculated for transverse microtubules in
treated samples compared with 1/2 Murashige and Skoog medium (pH 5.8) growing roots used as controls (**_P_ < 0.001). I–K, Auxin distribution approximated by DII-VENUS at the lower side
(LS) and upper side (US) of 90° re-oriented WT root (in DII-VENUS background). Enlarged pictures (I) are shown as the frames highlighted (K). Signal intensity is represented by the colour
code as indicated. The relative signal for the upper side and lower side (J) is expressed compared with the signal in the respective frame before gravistimulation. Student’s _t_-test was
calculated for the signal between the upper and lower sides at each time point (**_P_ < 0.001). In all panels, average values are shown and error bars are s.e.m. Scale bars, 5 μm (B, D,
E, G) and 30 μm (K). EXTENDED DATA FIGURE 2 FUNCTIONAL INACTIVATION OF ABP1 RESULTS IN MICROTUBULE DEFECTS GRADUALLY INCREASING WITH TIME OF ABP1 INACTIVATION. A, B, MAP4–GFP visualization
of microtubule orientation in WT and _tir1-1 afb1-1 afb2-1 afb3-1_ (abbreviated as _tir1afb1,2,3_) seedlings following 100 nM NAA treatment for 60 min. The proportion of cells with the four
categories of microtubule orientation patterns was determined, and Student’s _t_-test was calculated for the category of transverse microtubule compared with WT treated in the same condition
(**_P_ < 0.001). C–F, MAP4–GFP visualization and quantification of microtubule orientation in roots (C, D) or dark-grown hypocotyls (E, F) of WT, SS12S and SS12K seedlings following
different times of ethanol induction as indicated. Student’s _t_-test was calculated for the transverse microtubules compared with WT exposed for the same time to ethanol vapours as the
conditional ABP1 lines (*_P_ < 0.05, **_P_ < 0.001). In all panels, average values are shown and error bars are s.e.m. Scale bars, 5 μm (A, C) and 10 μm (E). EXTENDED DATA FIGURE 3
ABP1 IS INVOLVED IN MICROTUBULE REARRANGEMENT FOLLOWING GRAVISTIMULATION. A, Rearrangement of microtubules at the lower side compared with the upper side of 90° re-oriented roots of WT,
SS12S, SS12K and _abp1-5_ (all expressing MAP4–GFP). Two different types of microtubule orientation (90 ± 30° or 0–60°/120–180°) were quantified. Student’s _t_-test was calculated for the
category of transverse microtubules compared with each 0′ time point and calculated for transverse microtubules in the lower side compared with the upper side at each time point (**_P_ <
0.001). B, C, Auxin distribution simulated by DII-VENUS at the lower side compared with the upper side of 90° re-oriented roots of SS12S and SS12K (all in DII-VENUS background; enlarged
pictures were visualized in the frames highlighted). Image stacks were taken every 10 minutes, in total for 60 minutes. The ratio of the lower side signal divided by that of the upper side
is shown in the chart (C). Student’s _t_-test was calculated for the signal ratio at each time point of SS12S/K compared with WT (**_P_ < 0.001). Signal intensity is represented by the
colour code as indicated. Data for SS12S and SS12K (B) are compared with WT (Extended Data Fig. 1i–k). D, The deviated angles of 90° gravistimulated-roots of WT, _abp1-5_, SS12S and SS12K
seedlings were calculated every 30 min, in total for 8 h (Student’s _t_-test, *_P_ < 0.05, **_P_ < 0.001). In all panels, average values are shown and error bars are s.e.m. Scale bars,
5 μm (A) and 30 μm (B). EXTENDED DATA FIGURE 4 THE EFFECT OF AUXIN ON FAST RESPONSIVENESS OF MICROTUBULE DYNAMICS IS DEPENDENT ON ABP1. A, B, Acquisition and quantification of the rate of
EB1b movement in roots of untreated or 100 nM NAA-treated (60 min) WT or SS12K (expressing EB1b–GFP) by measuring EB1b–GFP growth events as highlighted by red lines (Student’s _t_-test, _P_
> 0.05). Box plots indicate the 25th centile (bottom boundary), median (middle line), 75th centile (top boundary), the nearest observations within 1.5 times, the interquartile range and
outliers. C, EB1b movement was simulated as transverse (blue, 90 ± 30°) or longitudinal (red, 0–60°/120–180°) trajectories before (0′) and after (180′′) 100 nM NAA treatment in WT background
(colour maps). The blue/red surface ratio is quantified on the chart (_n_ = 5); C corresponds to Fig. 3a. D, Microtubule orientation patterns after 400 µM cordycepin plus NAA co-treatment.
Student’s _t_-test was calculated for the category of transverse microtubule compared with only cordycepin treatment (**_P_ < 0.001). E, EB1b trajectories (simulated by time-stack from 10
min videos) were visualized and quantified after DMSO, IAA (1 µM), PEO-IAA (10 µM) and PEO-IAA (10 µM) plus IAA (1 µM) treatments. The left panel shows successive frames of 90′ acquisitions
following IAA application of pre-treated PEO-IAA WT roots. Student’s _t_-test was calculated for the category of transverse microtubules compared with DMSO treatment at each time point
(**_P_ < 0.001). F–I, Projections of EB1b–GFP in SS12K roots (F) and quantification (G) from every 15 s acquisition during 10 min (Supplementary Videos 4 and 6) following DMSO or 100 nM
NAA application (_n_ = 10). Blue and red strips represent transverse (90 ± 30°) and oblique/longitudinal (0–60°/120–180°) directions, respectively (F). Colour maps show the simulated
transverse or longitudinal trajectories of EB1b before (0′) and after (180′′) 100 nM NAA treatment in SS12K (H) or SS12S (I) roots. The blue/red surface ratio is quantified on the charts
(_n_ = 5) (H, I). The data of SS12S (I) correspond to Fig. 3b, and the data of SS12S and SS12K (F–I) are compared with WT (Fig. 3a and Extended Data Fig. 4c). In all panels except B, average
values are shown, error bars are s.e.m. and scale bars are 5 μm. EXTENDED DATA FIGURE 5 OVEREXPRESSED ABP1-INDUCED EFFECT OF AUXIN ON FAST RESPONSIVENESS OF MICROTUBULE DYNAMICS. A–C, ABP1
and ABP1–GFP transcripts (A) and ABP1 protein level (B, C) were detected in WT and XVE ≫ ABP1-OE line before and after 2 µM oestradiol induction for 12 h or 48 h before RNA or protein
extraction. The transcript levels of ABP1 in WT with DMSO treatment were standardized as ‘1’ (A). The 22 kDa native ABP1 band and 49KDa ABP1–GFP band were detected and quantified in the
right chart. The protein level of native ABP1 or ABP1–GFP in WT was standardized as ‘1’ for each ABP1 and ABP1–GFP, respectively (B, C). Student’s _t_-test, **_P_ < 0.001. D, Time-lapse
observation of microtubule orientation in the roots of XVE ≫ ABP1-OE roots expressing TUA6–RFP, WT and _abp1-5_ (both expressing MAP4–GFP) upon 100 nM NAA treatment. The percentage of
re-oriented microtubules (0–60°/120–180°) was quantified. Re-oriented microtubules in the inducible XVE ≫ ABP1-OE TUA6–RFP roots were calculated compared with none-inducible roots, and
_abp1-5_ MAP4–GFP was compared with MAP4–GFP at each time point (Student’s _t_-test, *_P_ < 0.05, **_P_ < 0.001). In all panels, average values are shown, error bars are s.e.m and
scale bars are 5 μm. EXTENDED DATA FIGURE 6 CALCIUM STARVATION DISRUPTS MICROTUBULE ORIENTATION AND HIGH CALCIUM INCREASES MICROTUBULE DEPOLYMERIZATION. Orientation and polymerization
statuses of microtubules were visualized following transfer of seedlings to different concentrations of CaCl2 for 30, 90 or 180 min. Low calcium levels disrupted microtubule organization,
leading to a predominantly random pattern; high calcium caused microtubule depolymerization. Student’s _t_-test was calculated for the category of transverse microtubules compared with
seedlings grown and transferred on standard 1/2 Murashige and Skoog medium (with 1.5 mM CaCl2) (*_P_ < 0.05, **_P_ < 0.001). In all panels, average values are shown, error bars are
s.e.m. and scale bars are 5 μm. EXTENDED DATA FIGURE 7 AUXIN–ABP1 CONTROLS MICROTUBULE ARRANGEMENT THROUGH DOWNSTREAM ROP6–RIC1–KTN1 SIGNALLING. A, MAP4–GFP visualization of microtubule
orientation in the root of WT, _rop6-1_, _ric1-1_, SS12S _ric1-1_ and SS12K _ric1-1_ following DMSO application for 60 min. Pictures in A correspond to quantifications in Fig. 4a. B, C,
Microtubule re-orientation patterns were visualized by MAP4–GFP in the roots of WT and _rop6-1_+/− following DMSO or 100 nM NAA application for 60 min (Student’s _t_-test, _P_ > 0.05). D,
Transcript level of the _scFv12_ coding the recombinant antibody responsible for ABP1 knockdown in WT, _ric1-1_, _ktn1_, SS12S, SS12K, SS12S _ric1-1_, SS12K _ric1-1_, SS12S _ktn1_ and SS12K
_ktn1_ after 48 h ethanol induction. The transcript level of the _scFv12_ in SS12S was standardized as ‘1’ (Student’s _t_-test, _P_ > 0.05). E, Microtubule orientation by MAP4–GFP in
dark-grown hypocotyls of WT, SS12K, _ktn1_ and SS12K _ktn1_ (with 24 h ethanol induction) following DMSO application for 60 min. Pictures in e correspond to Fig. 4b. In all panels, average
values are shown and error bars are s.e.m. Scale bars, 5 μm (A, B) and 10 μm (E). SUPPLEMENTARY INFORMATION THE TRAJECTORIES OF EB1B IN WT BACKGROUND FOLLOWING DMSO TREATMENT EB1b-GFP
seedlings were mounted on DMSO-contained 1/2 MS glass slides and imaged immediately for 10min. EB1b-GFP comets illustrate major transversal MT growth trajectories (90±30°). Corresponding to
Fig. 3a. (MOV 3725 kb) THE TRAJECTORIES OF EB1B IN WT BACKGROUND FOLLOWING 100NM NAA TREATMENT EB1b-GFP seedlings were mounted on 1/2 MS glass slides containing 100nM NAA and imaged
immediately for 10min. EB1b-GFP moves mainly along 90±30° transversal direction in the beginning (0-60sec), while increasing EB1b tracks switch along oblique/longitudinal direction
(0-60°/120-180°) after 75sec. Corresponding to Fig. 3a. (MOV 2184 kb) THE TRAJECTORIES OF EB1B IN ABP1 KNOCKDOWN LINES FOLLOWING DMSO TREATMENT 48h ethanol induced SS12S or SS12K seedlings
expressing EB1b-GFP were mounted on DMSO-contained 1/2 MS glass slides and imaged immediately for 10min. High proportion of EB1b-GFP moves in oblique/longitudinal directions. Corresponding
to Fig. 3b, Extended Data Fig. 4d. (MOV 1813 kb) THE TRAJECTORIES OF EB1B IN ABP1 KNOCKDOWN LINES FOLLOWING DMSO TREATMENT 48h ethanol induced SS12S or SS12K seedlings expressing EB1b-GFP
were mounted on DMSO-contained 1/2 MS glass slides and imaged immediately for 10min. High proportion of EB1b-GFP moves in oblique/longitudinal directions. Corresponding to Fig. 3b, Extended
Data Fig. 4d. (MOV 1383 kb) THE TRAJECTORIES OF EB1B IN SS12S OR SS12K BACKGROUND FOLLOWING 100NM NAA TREATMENT 48h ethanol induced SS12S or SS12K seedlings expressing EB1b-GFP were mounted
on 1/2 MS glass slides containing 100nM NAA and imaged immediately for 10min. Compared with WT, no consistent switch of EB1b trajectories to longitudinal directions but only few stochastic
changes were observed. Corresponding to Fig. 3b, Extended Data Fig. 4d. (MOV 1717 kb) THE TRAJECTORIES OF EB1B IN SS12S OR SS12K BACKGROUND FOLLOWING 100NM NAA TREATMENT 48h ethanol induced
SS12S or SS12K seedlings expressing EB1b-GFP were mounted on 1/2 MS glass slides containing 100nM NAA and imaged immediately for 10min. Compared with WT, no consistent switch of EB1b
trajectories to longitudinal directions but only few stochastic changes were observed. Corresponding to Fig. 3b, Extended Data Fig. 4d. (MOV 2100 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR
FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chen, X.,
Grandont, L., Li, H. _et al._ Inhibition of cell expansion by rapid ABP1-mediated auxin effect on microtubules. _Nature_ 516, 90–93 (2014). https://doi.org/10.1038/nature13889 Download
citation * Received: 12 November 2013 * Accepted: 23 September 2014 * Published: 17 November 2014 * Issue Date: 04 December 2014 * DOI: https://doi.org/10.1038/nature13889 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative
