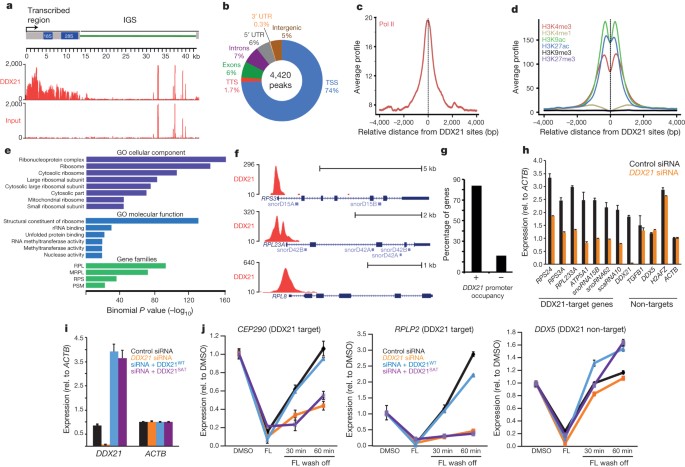
Rna helicase ddx21 coordinates transcription and ribosomal rna processing
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT DEAD-box RNA helicases are vital for the regulation of various aspects of the RNA life cycle1, but the molecular underpinnings of their involvement, particularly in mammalian cells,
remain poorly understood. Here we show that the DEAD-box RNA helicase DDX21 can sense the transcriptional status of both RNA polymerase (Pol) I and II to control multiple steps of ribosome
biogenesis in human cells. We demonstrate that DDX21 widely associates with Pol I- and Pol II-transcribed genes and with diverse species of RNA, most prominently with non-coding RNAs
involved in the formation of ribonucleoprotein complexes, including ribosomal RNA, small nucleolar RNAs (snoRNAs) and 7SK RNA. Although broad, these molecular interactions, both at the
chromatin and RNA level, exhibit remarkable specificity for the regulation of ribosomal genes. In the nucleolus, DDX21 occupies the transcribed rDNA locus, directly contacts both rRNA and
snoRNAs, and promotes rRNA transcription, processing and modification. In the nucleoplasm, DDX21 binds 7SK RNA and, as a component of the 7SK small nuclear ribonucleoprotein (snRNP) complex,
is recruited to the promoters of Pol II-transcribed genes encoding ribosomal proteins and snoRNAs. Promoter-bound DDX21 facilitates the release of the positive transcription elongation
factor b (P-TEFb) from the 7SK snRNP in a manner that is dependent on its helicase activity, thereby promoting transcription of its target genes. Our results uncover the multifaceted role of
DDX21 in multiple steps of ribosome biogenesis, and provide evidence implicating a mammalian RNA helicase in RNA modification and Pol II elongation control. Access through your institution
Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and
online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes
which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY
OTHERS DEAD-BOX ATPASE DBP2 IS THE KEY ENZYME IN AN MRNP ASSEMBLY CHECKPOINT AT THE 3’-END OF GENES AND INVOLVED IN THE RECYCLING OF CLEAVAGE FACTORS Article Open access 09 August 2024 THE
CONSERVED RNA-BINDING PROTEIN SEB1 PROMOTES COTRANSCRIPTIONAL RIBOSOMAL RNA PROCESSING BY CONTROLLING RNA POLYMERASE I PROGRESSION Article Open access 25 May 2023 THE NUCLEOLAR SHELL
PROVIDES ANCHORING SITES FOR DNA UNTWISTING Article Open access 23 January 2024 ACCESSION CODES PRIMARY ACCESSIONS GENE EXPRESSION OMNIBUS * GSE56802 DATA DEPOSITS All sequencing data have
been deposited in Gene Expression Omnibus (GEO) data repository under accession number GSE56802. REFERENCES * Rocak, S. & Linder, P. DEAD-box proteins: the driving forces behind RNA
metabolism. _Nature Rev. Mol. Cell Biol._ 5, 232–241 (2004) Article CAS Google Scholar * Russell, R., Jarmoskaite, I. & Lambowitz, A. M. Toward a molecular understanding of RNA
remodeling by DEAD-box proteins. _RNA Biol._ 10, 44–55 (2013) Article CAS Google Scholar * Putnam, A. A. & Jankowsky, E. DEAD-box helicases as integrators of RNA, nucleotide and
protein binding. _Biochim. Biophys. Acta_ 1829, 884–893 (2013) Article CAS Google Scholar * Henning, D., So, R. B., Jin, R., Lau, L. F. & Valdez, B. C. Silencing of RNA helicase
II/Guα inhibits mammalian ribosomal RNA production. _J. Biol. Chem._ 278, 52307–52314 (2003) Article CAS Google Scholar * Yang, H. et al. Down-regulation of RNA helicase II/Gu results in
the depletion of 18 and 28 S rRNAs in _Xenopus_ oocyte. _J. Biol. Chem._ 278, 38847–38859 (2003) Article CAS Google Scholar * Westermarck, J. et al. The DEXD/H-box RNA helicase RHII/Gu is
a co-factor for c-Jun-activated transcription. _EMBO J._ 21, 451–460 (2002) Article CAS Google Scholar * Zentner, G. E., Saiakhova, A., Manaenkov, P., Adams, M. D. & Scacheri, P. C.
Integrative genomic analysis of human ribosomal DNA. _Nucleic Acids Res._ 39, 4949–4960 (2011) Article CAS Google Scholar * Cong, R. et al. Interaction of nucleolin with ribosomal RNA
genes and its role in RNA polymerase I transcription. _Nucleic Acids Res._ 40, 9441–9454 (2012) Article Google Scholar * Dieci, G., Preti, M. & Montanini, B. Eukaryotic snoRNAs: a
paradigm for gene expression flexibility. _Genomics_ 94, 83–88 (2009) Article CAS Google Scholar * Chao, S. H. et al. Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. _J. Biol.
Chem._ 275, 28345–28348 (2000) Article CAS Google Scholar * Chao, S. H. & Price, D. H. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription _in vivo_. _J.
Biol. Chem._ 276, 31793–31799 (2001) Article CAS Google Scholar * Valdez, B. C., Henning, D., Perumal, K. & Busch, H. RNA-unwinding and RNA-folding activities of RNA helicase
II/Gu–two activities in separate domains of the same protein. _Eur. J. Biochem._ 250, 800–807 (1997) Article CAS Google Scholar * Perlaky, L., Valdez, B. C. & Busch, H. Effects of
cytotoxic drugs on translocation of nucleolar RNA helicase RH-II/Gu. _Exp. Cell Res._ 235, 413–420 (1997) Article CAS Google Scholar * Drygin, D. et al. Anticancer activity of CX-3543: a
direct inhibitor of rRNA biogenesis. _Cancer Res._ 69, 7653–7661 (2009) Article CAS Google Scholar * Huppertz, I. et al. iCLIP: Protein-RNA interactions at nucleotide resolution.
_Methods_ 65, 274–287 (2014) Article CAS Google Scholar * Zarnack, K. et al. Direct competition between hnRNP C and U2AF65 protects the transcriptome from the exonization of Alu elements.
_Cell_ 152, 453–466 (2013) Article CAS Google Scholar * Lui, L. & Lowe, T. Small nucleolar RNAs and RNA-guided post-transcriptional modification. _Essays Biochem._ 54, 53–77 (2013)
Article CAS Google Scholar * Hughes, J. M. & Ares, M., Jr Depletion of U3 small nucleolar RNA inhibits cleavage in the 5′ external transcribed spacer of yeast pre-ribosomal RNA and
impairs formation of 18S ribosomal RNA. _EMBO J._ 10, 4231–4239 (1991) Article CAS Google Scholar * Fatica, A., Galardi, S., Altieri, F. & Bozzoni, I. Fibrillarin binds directly and
specifically to U16 box C/D snoRNA. _RNA_ 6, 88–95 (2000) Article CAS Google Scholar * Newman, D. R., Kuhn, J. F., Shanab, G. M. & Maxwell, E. S. Box C/D snoRNA-associated proteins:
two pairs of evolutionarily ancient proteins and possible links to replication and transcription. _RNA_ 6, 861–879 (2000) Article CAS Google Scholar * Petfalski, E., Dandekar, T., Henry,
Y. & Tollervey, D. Processing of the precursors to small nucleolar RNAs and rRNAs requires common components. _Mol. Cell. Biol._ 18, 1181–1189 (1998) Article CAS Google Scholar * Yu,
Y. T., Shu, M. D. & Steitz, J. A. A new method for detecting sites of 2′-O-methylation in RNA molecules. _RNA_ 3, 324–331 (1997) CAS PubMed PubMed Central Google Scholar * Yang, Z.,
Zhu, Q., Luo, K. & Zhou, Q. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. _Nature_ 414, 317–322 (2001) Article ADS CAS Google Scholar * Ji,
X. et al. SR proteins collaborate with 7SK and promoter-associated nascent RNA to release paused polymerase. _Cell_ 153, 855–868 (2013) Article CAS Google Scholar * McNamara, R. P.,
McCann, J. L., Gudipaty, S. A. & D’Orso, I. Transcription factors mediate the enzymatic disassembly of promoter-bound 7SK snRNP to locally recruit P-TEFb for transcription elongation.
_Cell Rep._ 5, 1256–1268 (2013) Article CAS Google Scholar * Castelo-Branco, G. et al. The non-coding snRNA 7SK controls transcriptional termination, poising, and bidirectionality in
embryonic stem cells. _Genome Biol._ 14, R98 (2013) Article Google Scholar * Nguyen, V. T., Kiss, T., Michels, A. A. & Bensaude, O. 7SK small nuclear RNA binds to and inhibits the
activity of CDK9/cyclin T complexes. _Nature_ 414, 322–325 (2001) Article ADS CAS Google Scholar * Peterlin, B. M. & Price, D. H. Controlling the elongation phase of transcription
with P-TEFb. _Mol. Cell_ 23, 297–305 (2006) Article CAS Google Scholar * Michels, A. A. et al. Binding of the 7SK snRNA turns the HEXIM1 protein into a P-TEFb (CDK9/cyclin T) inhibitor.
_EMBO J._ 23, 2608–2619 (2004) Article CAS Google Scholar * Liu, W. et al. Brd4 and JMJD6-associated anti-pause enhancers in regulation of transcriptional pause release. _Cell_ 155,
1581–1595 (2013) Article CAS Google Scholar * Flynn, R. A., Almada, A. E., Zamudio, J. R. & Sharp, P. A. Antisense RNA polymerase II divergent transcripts are P-TEFb dependent and
substrates for the RNA exosome. _Proc. Natl Acad. Sci. USA_ 108, 10460–10465 (2011) Article ADS CAS Google Scholar * Wassarman, D. A. & Steitz, J. A. Structural analyses of the 7SK
ribonucleoprotein (RNP), the most abundant human small RNP of unknown function. _Mol. Cell. Biol._ 11, 3432–3445 (1991) Article CAS Google Scholar * Sharma, A. et al. The Werner syndrome
helicase is a cofactor for HIV-1 long terminal repeat transactivation and retroviral replication. _J. Biol. Chem._ 282, 12048–12057 (2007) Article CAS Google Scholar * McLean, C. Y. et
al. GREAT improves functional interpretation of cis-regulatory regions. _Nature Biotechnol._ 28, 495–501 (2010) Article CAS Google Scholar * Carey, M. F., Peterson, C. L. & Smale, S.
T. Dignam and Roeder nuclear extract preparation. _Cold Spring Harb. Protoc._ 2009 10.1101/pdb.prot5330 (2009) * Konig, J. et al. iCLIP–transcriptome-wide mapping of protein–RNA interactions
with individual nucleotide resolution. _J. Vis. Exp._ 50, 2638 (2011) Google Scholar * Krueger, B. J., Varzavand, K., Cooper, J. J. & Price, D. H. The mechanism of release of P-TEFb
and HEXIM1 from the 7SK snRNP by viral and cellular activators includes a conformational change in 7SK. _PLoS ONE_ 5, e12335 (2010) Article ADS Google Scholar Download references
ACKNOWLEDGEMENTS We thank D. H. Price for the LARP7 antibody, K. Lane from M. Covert’s laboratory for metabolic inhibitors, K. Cimprich and members of the Chang and Wysocka laboratories for
discussions, and B. Zarnegar and P. Khavari for discussions regarding iCLIP. This work was supported by the Stanford Medical Scientist Training Program and T32CA09302 (R.A.F.), AP Giannini
Foundation (R.C.S.), National Institutes of Health grants R01-HG004361, R01-ES023168, P50-HG007735 (H.Y.C.) and R01-GM095555 (J.W.), W. M. Keck Foundation (J.W.), and Helen Hay Whitney
Foundation (E.C.). H.Y.C. is an Early Career Scientist of the Howard Hughes Medical Institute. AUTHOR INFORMATION Author notes * Eliezer Calo and Ryan A. Flynn: These authors contributed
equally to this work. AUTHORS AND AFFILIATIONS * Department of Chemical and Systems Biology, Stanford University School of Medicine, Stanford, 94305, California, USA Eliezer Calo &
Joanna Wysocka * Howard Hughes Medical Institute and Program in Epithelial Biology, Stanford University School of Medicine, Stanford, 94305, California, USA Ryan A. Flynn, Lance Martin,
Robert C. Spitale & Howard Y. Chang * Department of Developmental Biology, Stanford University School of Medicine, Stanford, 94305, California, USA Joanna Wysocka Authors * Eliezer Calo
View author publications You can also search for this author inPubMed Google Scholar * Ryan A. Flynn View author publications You can also search for this author inPubMed Google Scholar *
Lance Martin View author publications You can also search for this author inPubMed Google Scholar * Robert C. Spitale View author publications You can also search for this author inPubMed
Google Scholar * Howard Y. Chang View author publications You can also search for this author inPubMed Google Scholar * Joanna Wysocka View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS H.Y.C. and J.W. supervised the project; E.C. and R.A.F. conceived and designed the study; E.C. and R.A.F. performed experiments and analysed
ChIP-seq data. R.A.F. performed iCLIP and L.M., R.C.S. and R.A.F. analysed iCLIP data; R.A.F., E.C., J.W. and H.Y.C. wrote the manuscript with input from all co-authors. CORRESPONDING
AUTHORS Correspondence to Howard Y. Chang or Joanna Wysocka. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. EXTENDED DATA FIGURES AND TABLES
EXTENDED DATA FIGURE 1 DDX21 ASSOCIATES WITH NON- AND PROTEIN-CODING RIBOSOMAL GENES. A, MEME analysis of DDX21-bound regions defined by DDX21 ChIP-seq. Motif logo, annotated transcription
factor, number of motif instances within the ChIP-seq regions, _Z_ score, and _P_ value for each motif are shown. B, DDX21 ChIP-qPCR from HeLa cell chromatin extracts with primers spanning a
representative number of loci found to be enriched in the DDX21 ChIP-seq analyses from HEK293 cells. Data are mean and s.d. of three independent experiments. C, Comparison of DDX21 (this
study) and H3K4me3 (publically available data, see Methods for accession numbers) ChIP-seq-bound regions. 2,863 regions are common between the data sets, 505 regions are unique to DDX21, and
11,403 regions are unique to the H3K4me3 data set. D, Gene Ontology terms for H3K4me3 regions that are either DDX21-bound (left) or not bound by DDX21 (right). E, Box plots representing the
expression levels of snoRNA-host genes whose promoter regions are either bound or not by DDX21. As shown, snoRNA-host gene promoters bound by DDX21 are, on average, more highly expressed
than those not occupied by DDX21. Fragments per kilobase of exon per million mapped reads (FPKM) values were taken from publically available HEK293 RNA-seq data (see Methods for accession
number). The _P_ value (_P_ ≤ 0.05) was calculated using the Wilcoxon signed-rank test. F, G, UCSC genome browser tracks depicting DDX21 ChIP-seq and iCLIP-seq, and RNA-seq enrichment
profiles at differentially expressed snoRNA-host genes in HEK293 cells. EXTENDED DATA FIGURE 2 DDX21 POSITIVELY REGULATES TRANSCRIPTION OF POL I- AND POL II-DEPENDENT RIBOSOMAL GENES. A,
siRNA-mediated knockdown of the DDX21 antibody used for ChIP. We transfected HEK293 cells with two different sets of siRNAs targeting endogenous _DDX21_ mRNA (siRNA1 and siRNA2 (3′ UTR)) and
performed western blots with the indicated antibodies. As shown, the DDX21-specific band is diminished in cells transfected with _DDX21_-targeting siRNAs, but not with control siRNAs. Actin
was used as a loading control for this experiment. B, RT–qPCR analysis assessing the RNA expression levels of the same genes analysed in Fig. 1h upon _DDX21_ knockdown by a second siRNA
that targets the 3′ UTR of _DDX21_ mRNA. Data are mean and s.d. of three independent experiments. For DDX21-target genes the difference between control and _DDX21_ siRNA is significant, _P_
≤ 0.05 (Student’s _t_-test). C, Diagram of DDX21 protein domains. The two conserved RecA-like (A and B) domains and the GUCT domains are shown in green and blue, respectively. Amino acids
targeted for mutation12 to convert DDX21WT into DDX21SAT, the ATP-hydrolysis mutant, are indicated with red and purple lines in the diagram. Specific amino acid changes are displayed below.
D, qRT–PCR analysis assessing nascent unspliced mRNA levels from additional DDX21-target and DDX21-non-target promoters. For a detailed description see Fig. 1j. Data are mean and s.d. of
three biological replicates. E, Nuclear rRNA abundance analysis by RNA BioAnalyzer of HEK293 cells depleted of DDX21 and rescued with DDX21WT, DDX21SAT, or DDX21DEV. For each analysis, total
RNA was isolated from 1,500,000 nuclei. Total nanogram amounts are shown for each of the two large rRNA subunits. EXTENDED DATA FIGURE 3 SELECTIVE INHIBITION OF POL I ALTERS DDX21 NUCLEAR
LOCALIZATION AND CHROMATIN ASSOCIATION. A, B, Immunofluorescence images of methanol-fixed HEK293 cells after 1 h incubation with either DMSO or 2 μM of the specific Pol I inhibitor CX-5461.
DDX21 (A) and fibrillarin (B) immuno-labellings are shown. Scale bars, 10 μm. C, D, ChIP-qPCR analyses from HEK293 sampling DDX21 genomic occupancy, at the rDNA locus (C) and at a
representative panel of Pol II-regulated gene promoters (D), after treatment with DMSO or CX-5461. Data are mean and s.d. of three independent experiments. As displayed, inhibition of Pol I
alters DDX21 nuclear localization and this coincides with nearly complete eviction of DDX21 from Pol I- and Pol II-regulated genes. E, F, ChIP-qPCR analyses from HEK293 cells treated with 50
ng ml−1 of actinomycin-D for 1 h. Binding of the transcriptional repressor CTCF across the rDNA locus (E) and the c-MYC insulator element (MINE) (F) demonstrates that actinomycin-D
treatment does not effect CTCF binding to chromatin. Red arrow indicates relative location of the CTCF DNA-binding site (DBS) at the rDNA locus. Data are mean and s.d. of three independent
experiments. EXTENDED DATA FIGURE 4 DDX21 NUCLEAR RE-LOCALIZATION IS PREFERENTIALLY SENSITIVE TO ACUTE TRANSCRIPTIONAL INHIBITION OVER OTHER CELLULAR STRESSORS. Immunofluorescence analyses
of HEK293 cells after targeting different metabolic pathways. For inhibition of mitogen, cells were starved for 16 h in the absence of serum. For cellular respiration inhibition, cells were
treated for with either oligomycin (100 μM) or 2-deoxy-d-glucose (10 mM) for 1 h. To inhibit the mTOR pathway, cells were treated with 250 nM of either Torin 1 or rapamycin for 2 h. EXTENDED
DATA FIGURE 5 TANDEM AFFINITY ICLIP OF FH–DDX21WT. A, FH–DDX21WT iCLIP 32P-autoradiogram and western blots. All samples were loaded with constant input lysate amounts (actin loading).
FH–DDX21WT was isolated from HEK293 cells induced to express the transgene for 24 h and crosslinked with ultraviolet light (top panel same as Fig. 3a). B, Schematic of the modified iCLIP
procedure. To achieve high stringency and specificity Flag–HA–DDX21WT is first purified on anti-Flag–M2 agarose beads, washed with 1 M NaCl, 1% Triton X-100 and 1% sodium deoxycholate.
Complexes are specifically eluted with Flag peptide and recaptured with anti-HA agarose. Standard iCLIP steps were performed thereafter to generate deep sequencing libraries. C–E, Scatter
plot analysis of iCLIP reverse transcription stops on snoRNAs, rRNA and mRNAs within the FH–DDX21WT (this study) and hnRNP-C (ref. 16; publically available data) data sets. Little
concordance between the data sets is evident, suggesting specific transcriptome targets of these two RNA binding proteins (RBPs). F, DDX21WT ultraviolet RNA immunoprecipitation qRT–PCR of
FH–DDX21WT was performed in three conditions: native HEK293 cells crosslinked with ultraviolet light; FH–DDX21WT HEK293 cells without crosslinking; and FH–DDX21WT HEK293 cells with
ultraviolet crosslinking. snoRNAs, scaRNAs and TERC were validated targets identified in the sequencing data. Each experiment was performed in biological duplicates (rep1 and rep2) and error
bars represent s.d. of technical triplicates. EXTENDED DATA FIGURE 6 TANDEM AFFINITY ICLIP OF FH–DDX21SAT. A, FH–DDX21SAT was isolated from HEK293 cells induced to express the transgene for
24 h, at which point we did not observe significant dominant negative effects. iCLIP was performed as described for FH–DDX21WT, and biological duplicates of FH–DDX21SAT iCLIP
32P-autoradiogram and western blots (lanes 2 and 3) are shown. All samples were loaded with constant input lysate amounts (actin loading). FH–DDX21WT was loaded as a control. WB, western
blot. B, Left, DDX21SAT iCLIP reads annotated to known repetitive (rRNA and snRNAs) and non-repetitive (hg19 genome build: mRNAs and snoRNAs) regions of the human genome. Categories are
notes with their respective percentage of the total iCLIP experiment. Right, enriched Gene Ontology and KEGG pathway terms from DDX21SAT-bound mRNAs obtained using the DAVID tool. The _x_
axis values (in log scale) correspond to the negative Benjamini _P_ value. C, Distribution of all DDX21SAT-bound snoRNAs, representing C/D box, H/ACA box and scaRNAs. The number (_n_) and
fraction (per cent) of each snoRNA type is displayed. D, Comparison of the snoRNAs bound by DDX21WT and DDX21SAT, revealing significant overlap between the active and catalytically inactive
DDX21. E, DDX21SAT iCLIP reads mapped to the transcribed region of the rDNA. F, DDX21WT (left) and DDX21SAT (right) iCLIP reads mapped to the repetitive U3 snoRNA. Binding is represented as
reverse transcription stops per nucleotide normalized to the total number of reverse transcription stops mapping to the U3 snoRNA. Two strong binding sites are evident between bases 25–40
and 175–185 of U3 in DDX21WT iCLIP, whereas the 5′ binding site is reduced in DDX21SAT. nts, nucleotides. G, qRT–PCR analysis assessing the expression levels of several snoRNAs 24 h after
expression of either DDX21WT or DDX21SAT. This experiment was performed in biological duplicates. Data are mean and s.d. of technical triplicates. EXTENDED DATA FIGURE 7 DDX21 FUNCTIONALLY
INTERACTS WITH SNORNAS AND THE SNORNP. A, UCSC genome browser view of DDX21WT iCLIP reads across the snorD66 snoRNA. The C box [C] and D box [D] regions are highlighted in red. B, Same
visualization as in A but showing the snorA67 snoRNA with the H box [H] and ACA box [ACA] regions highlighted. C, Immunoprecipitation of NOP58 from HEK293 nuclear extracts confirms DDX21 as
a protein member of the snoRNP machinery. As a control for this experiment we performed western blots against FBL, a well-known NOP58-interacting partner and an essential factor of the
snoRNP machinery. D, DDX21 interacts with XRN2, a 5′–3′ exoribonuclease required for maturation and processing of snoRNAs. The DDX21–XRN2 interaction appears to be bridged by RNA, as
treatment of the nuclear lysates with RNaseA abolishes the interaction. E, Schematic of the site-directed RNaseH cleavage of RNA sensitive to 2′-_O_me. RNA of interest is hybridized to a
2′-_O_me/DNA chimaeric oligonucleotide in which the DNA nucleotides specifically target the ability of RNaseH to interrogate the 2′-_O_me status of a single nucleotide. 2′-_O_me will inhibit
RNaseH and leave intact RNA, while unmethylated RNA will be cleaved. F, UCSC genome browser view of DDX21WT and DDX21SAT iCLIP reads across the snoRNAs responsible for guiding the
modifications tested in Fig. 3f. EXTENDED DATA FIGURE 8 ASSOCIATION OF DDX21 WITH THE RNA AND PROTEIN COMPONENTS OF THE 7SK SNRNP. A, Comparison of DDX21WT ChIP-seq targets to DDX21WT
iCLIP-bound mRNAs. The numbers of unique and common genes are represented, revealing that most ChIP-seq-bound genes are not immunoprecipitated by iCLIP but that some are recovered in both
assays. B, iCLIP read distribution of DDX21WT-target mRNAs categorized by the regions within mRNAs that were bound. Most iCLIP reads fell outside the 5′ UTR. C, DDX21WT iCLIP reads mapping
to short repetitive RNAs of the human genome. Percentages of the top four short repetitive RNAs are shown. D, Secondary structure model of the 7SK snRNA annotated with iCLIP reverse
transcription stops identified from the DDX21WT (blue) and DDX21SAT (orange) experiments. Nucleotides commonly crosslinked are labelled in green. Known RNA binding protein sites: HEXIM1/2 is
highlighted in purple; P-TEFb is highlighted in red; and other P-TEFb ‘release’ factors in the centre are highlighted in green. E, Co-immunoprecipitation analysis of HEXIM1 as assayed by
western blotting for DDX21WT and 7SK snRNP components (CDK9 and LARP7). F, Immunoprecipitation of Flag–HA–DDX21WT from HEK293 nuclear extracts confirms DDX21 as a protein component of the
7SK snRNP through co-recovery of LARP7. The abundant protein actin, which is not part of the 7SK snRNP, was not recovered. EXTENDED DATA FIGURE 9 BINDING OF THE DDX21–7SK SNRNP AT RIBOSOMAL
GENE PROMOTERS. A, B, ChIP-qPCR of CDK9 (A) and HEXIM1 (B) in HEK293 cells at representative Pol II-regulated, DDX21-target and -non-target gene promoters, negative control regions, and the
rDNA locus. C, ChIP-qPCR of DDX21 in control or 3′-7SK-ASO-treated HEK293 cells at representative Pol II-regulated, DDX21-target gene promoters, negative control regions, and the rDNA locus.
For the promoter-associated genes, _P_ ≤ 0.05 (Student’s _t_-test) when compared to control ASO. D, Gene Ontology molecular function and cellular component analysis of publically available
CDK9 ChIP-seq data30. E, ChIP-qPCR of total Pol II in control or DDX21-targeting siRNA-treated HEK293 cells at representative TSSs of Pol II-regulated, DDX21-target gene promoters and
negative control regions. Data are mean and s.d. of three independent experiments. EXTENDED DATA FIGURE 10 CATALYTICALLY INACTIVE DDX21 IS STILL INCORPORATED INTO THE 7SK SNRNP. A, ChIP-qPCR
of DDX21WT (black) and DDX21SAT (green) in HEK293 cells at representative TSSs of Pol II-regulated, DDX21-target gene promoters and negative control regions. Data are mean and s.d. of three
independent experiments. B, Immunoprecipitation of DDX21WT, DDX21DEV or DDX21SAT from HEK293 nuclear extracts confirms DDX21 interacts with CDK9 (P-TEFb) regardless of its catalytic
activity. C, DDX21WT (blue) and DDX21SAT (orange) annotated iCLIP reads mapped across the 7SK snRNA. The four annotated stem–loops are marked below the graph. D, Model of multi-level control
of ribosomal pathway by DDX21. In the nucleolus, DDX21 associates with the chromatin across the transcribed region of the rDNA and is a component of the snoRNP. Furthermore, DDX21
functionally interacts with the rRNA, snoRNAs and snoRNP to control 2′-_O_me deposition on the rRNA in a helicase activity-dependent manner. In the nucleoplasm, DDX21 is bound to the
promoter regions of ribosomal Pol II-transcribed genes, many of which contain precursor snoRNA transcripts. Mechanistically, DDX21 activates transcription of its target genes through the
7SK–P-TEFb axis. As part of the 7SK snRNP, DDX21 can facilitate the release of P-TEFb from the inhibitory complex in a manner dependent on ATP hydrolysis, leading to productive Pol II
elongation and increased phosphorylation of Ser 2. Efficient transcription of its target genes enforces high expression of both snoRNAs and other ribosomal proteins critical for the rRNA
maturation process, placing DDX21 as a central operator of the ribosomal pathway. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE I This fie contains oligonucleotides utilized in this study.
(XLSX 51 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Calo, E., Flynn, R., Martin, L. _et al._ RNA helicase DDX21 coordinates transcription and ribosomal RNA processing. _Nature_ 518, 249–253 (2015).
https://doi.org/10.1038/nature13923 Download citation * Received: 04 April 2014 * Accepted: 06 October 2014 * Published: 24 November 2014 * Issue Date: 12 February 2015 * DOI:
https://doi.org/10.1038/nature13923 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
