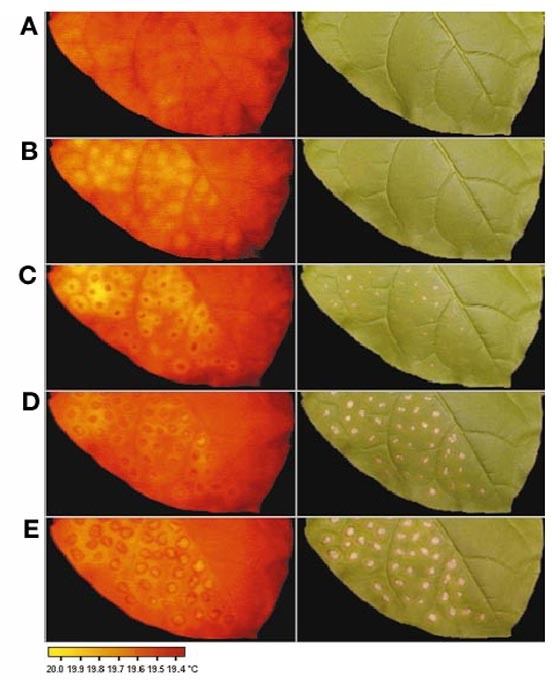
Presymptomatic visualization of plant–virus interactions by thermography
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Salicylic acid (SA), produced by plants as a signal in defense against pathogens, induces metabolic heating mediated by alternative respiration in flowers of thermogenic plants,
and, when exogenously applied, increases leaf temperature in nonthermogenic plants. We have postulated that the latter phenomenon would be detectable when SA is synthesized locally in plant
leaves. Here, resistance to tobacco mosaic virus (TMV) was monitored thermographically before any disease symptoms became visible on tobacco leaves. Spots of elevated temperature that were
confined to the place of infection increased in intensity from 8 h before the onset of visible cell death, and remained detectable as a halo around the ongoing necrosis. Salicylic acid
accumulates during the prenecrotic phase in TMV-infected tobacco and is known to induce stomatal closure in certain species. We show that the time course of SA accumulation correlates with
the evolution of both localized thermal effect and stomatal closure. Since the contribution of leaf respiration is marginal, we concluded that the thermal effect results predominantly from
localized, SA-induced stomatal closure. The presymptomatic temperature increase could be of general significance in incompatible plant–pathogen interactions. Access through your institution
Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and
online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes
which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY
OTHERS INTERPLAY BETWEEN DROUGHT AND PLANT VIRUSES CO-INFECTING MELON PLANTS Article Open access 09 July 2024 LIGHT PREVENTS PATHOGEN-INDUCED AQUEOUS MICROENVIRONMENTS VIA POTENTIATION OF
SALICYLIC ACID SIGNALING Article Open access 09 February 2023 NEW EARLY PHENOTYPIC MARKERS FOR CUCUMBER GREEN MOTTLE MOSAIC VIRUS DISEASE IN CUCUMBERS EXPOSED TO FLUCTUATING EXTREME
TEMPERATURES Article Open access 24 September 2021 REFERENCES * Malamy, J., Carr, J.P., Klessig, D.F. & Raskin, I. Salicylic acid: a likely endogenous signal in the resistance response
of tobacco to viral infection. _Science_ 250,1002– 1004 (1990). Article CAS Google Scholar * Métraux, J.P. et al. Increase in salicylic acid at the onset of systemic acquired resistance
in cucumber. _Science_ 250, 1004– 1006 (1990). Article Google Scholar * Uknes, S. et al. Regulation of pathogenesis-related protein-1a gene expression in tobacco. _Plant Cell_ 5, 159–169
(1993). Article CAS Google Scholar * Raskin, I., Turner, I.M. & Melander, W.R. Regulation of heat production in the inflorescences of an Arum lily by endogenous salicylic acid. _Proc.
Natl. Acad. Sci. USA_ 86, 2214–2218 ( 1989). Article CAS Google Scholar * Larqué-Saavedra, A. Stomatal closure in response to acetylsalicylic acid treatment. _ Z. Pflanzenphysiol._ 93,
371–375 (1979). Article Google Scholar * Manthe, B., Schulz, M. & Schnabl, H. Effects of salicylic acid on growth and stomatal movements of Vicia faba L.: evidence for salicylic acid
metabolization. _ J. Chem. Ecol._ 18, 1525–1539 (1992). Article CAS Google Scholar * Van Der Straeten, D., Chaerle, L., Sharkov, G., Lambers, H., & Van Montagu, M. Salicylic acid
enhances the activity of the alternative pathway of respiration in tobacco leaves and induces thermogenicity. _ Planta_ 196, 412–419 ( 1995). Article CAS Google Scholar * Malamy, J.,
Hennig, J. & Klessig, D.F. Temperature-dependent induction of salicylic acid and its conjugates during the resistance response to tobacco mosaic virus infection. _Plant Cell_ 4, 359– 366
(1992). Article CAS Google Scholar * Mur, L.A.J., Bi, Y.-M., Darby, R.M., Firek, S. & Draper, J. Compromising early salicylic acid accumulation delays the hypersensitive response and
increases viral dispersal during lesion establishment in TMV-infected tobacco. _Plant J._ 12, 1113 –1126 (1997). Article CAS Google Scholar * Enyedi, A.J., Yalpani, N., Silverman, P.
& Raskin, I. Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. _Proc. Natl. Acad. Sci. USA_ 89, 2480–2484
(1992). Article CAS Google Scholar * De Carolis, C., Conti, G.G. & Lechi, G.M. In _Proceedings of the 10th International Symposium on Remote Sensing and Environment._ 659–672
(Environmental Research Institute of Michigan, Ann Arbor, MI; 1975). Google Scholar * Yalpani, N., Silverman, P., Wilson, T.M.A., Kleier, D.A. & Raskin, I. Salicylic acid is a systemic
signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. _Plant Cell_ 3, 809–818 (1991). Article CAS Google Scholar * Friedrich, L., Vernooij, B., Gaffney, T.,
Morse, A. & Ryals, J. Characterization of tobacco plants expressing a bacterial salicylate hydroxylase gene. _Plant Mol. Biol._ 29:, 959–968 ( 1995). Article CAS Google Scholar *
Gaffney, T. et al. Requirement of salicylic acid for the induction of systemic acquired resistance. _Science_ 261, 754– 756 (1993). Article CAS Google Scholar * Lennon, A.M.,
Neuenschwander, U.H., Ribas-Carbo, M., Giles, L., Ryals, J.A., & Siedow, J.N. The effects of salicylic acid and tobacco mosaic virus infection on the alternative oxidase of tobacco.
_Plant Physiol. _ 115, 783–791 ( 1997). Article CAS Google Scholar * Chivasa, S., Murphy, A.M., Naylor, M. & Carr, J.P. Salicylic acid interferes with tobacco mosaic virus replication
via a novel salicylhydroxamic acid–sensitive mechanism. _Plant Cell_ 9, 547–557 (1997). Article CAS Google Scholar * Chivasa, S. & Carr, J. Cyanide restores N gene-mediated
resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic acid hydroxylase. _Plant Cell_ 10, 1489–1498 (1998). CAS PubMed PubMed Central Google Scholar * Day, D.A. et
al. The cyanide-resistant oxidase: to inhibit or not to inhibit, that is the question. _Plant Physiol._ 110, 1– 2 (1996). Article CAS Google Scholar * McNulty, A.K. & Cummins, W.R.
The relationship between respiration and temperature in leaves of the arctic plant Saxifraga cernua. _ Plant Cell Environ._ 10, 319–325 (1987). Article Google Scholar * Breidenbach, R.W.,
Saxton, M.J., Hansen, L.D. & Criddle, R.S. Heat generation and dissipation in plants: can the alternative oxidative phosphorylation pathway serve a thermoregulatory role in plant tissues
other than specialized organs? _Plant Physiol._ 114, 1137– 1140 (1997). Article CAS Google Scholar * Leung, J. & Giraudat, J. Abscisic acid signal transduction. Annu. Rev. Plant
Physiol. _Plant Mol. Biol._ 49, 199–222 (1998). CAS Google Scholar * Raskin, I. & Ladyman, J.A.R. Isolation and characterization of a barley mutant with abscisic acid–insensitive
stomata. _ Planta_ 173, 73–78 ( 1987). Article Google Scholar * Whenham, R.J. & Fraser, R.S.S. Effect of systemic and local-lesion-forming strains of tobacco mosaic virus on abscisic
acid acid concentration in tobacco leaves: consequences for the control of leaf growth. Physiol. _ Plant Pathol._ 18, 267–278 (1981). CAS Google Scholar * Weyers, J.D.B. & Meidner, H.
_Methods in stomatal research._ (Longman Scientific & Technical, Harlow, UK; 1990). Google Scholar * Nillsson, H.-E. Remote sensing and image analysis in plant pathology. _Ann Rev.
Phytopathol._ 15, 489–527 (1995). Article Google Scholar * Bachem, C.W.B., van der Hoeven, R.S., de BruiJn, S.M., Vreugdenhil, D., Zabeau, M., & Visser, R.G.F. Visualization of
differential gene expression using a novel method of RNA fingerprinting based on AFLP: analysis of gene expression during potato tuber development. _Plant J._ 9, 745–753 (1996). Article CAS
Google Scholar * Liang, P. & Pardee, A.B. 1997. Differential Display Methods and Protocols (_Methods in Molecular Biology_, Vol. 85) Humana Press, Totowa, NJ. Google Scholar * Bi.
Y–M., Kenton, P., Mur, L., Darby, R. & Draper, J. Hydrogen peroxide does not function downstream of saliclic acid in the induction of PR protein expression. _Plant J._, 8, 235–245
(1995). Article CAS Google Scholar * SAS/STAT Software. 1992. Changes and Enhancements, Release 6.07 (SAS Technical Report P-229), SAS Institute inc., Cary, NC. Download references
ACKNOWLEDGEMENTS We thank T.J. Pons and R. Welschen (Department of Plant Ecology and Evolutionary Biology, University of Utrecht, The Netherlands) for advice on energy-budget calculations
and steady state porometry and for help with respiratory and steady state porometrical measurements, respectively; O. Thas (Biomath Department, University of Gent, Belgium) for the
statistical analysis with SAS; R. Samson (Department of Applied Ecology and Environmental Biology, University of Gent) for advice on diffusion porometrical measurements; J. Ryals (Novartis,
Research Triangle Park, NC, USA) for providing the tobacco line NahG-10; and M. De Cock for lay-out. This research was supported by grants from the Fund for Scientific Research (Flanders)
(G.0023.95N and 1.5.514.98). L.C. was a Research Assistant and D.V.D.S. is a Research Associate of the Fund for Scientific Research (Flanders). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Laboratorium voor Genetica, Departement Plantengenetica, Vlaams Interuniversitair Instituut voor Biotechnologie, Universiteit Gent , K.L. Ledeganckstraat 35, Gent, B-9000, Belgium Laury
Chaerle, Wim Van Caeneghem, Eric Messens, Marc Van Montagu & Dominique Van Der Straeten * Plant Sciences, Faculty of Agriculture, The University of Western Australia, Nedland, 6907, WA,
Australia Hans Lambers * Department of Plant Ecology and Evolutionary Biology Utrecht University, Utrecht, NL-3584, CA, The Netherlands Hans Lambers Authors * Laury Chaerle View author
publications You can also search for this author inPubMed Google Scholar * Wim Van Caeneghem View author publications You can also search for this author inPubMed Google Scholar * Eric
Messens View author publications You can also search for this author inPubMed Google Scholar * Hans Lambers View author publications You can also search for this author inPubMed Google
Scholar * Marc Van Montagu View author publications You can also search for this author inPubMed Google Scholar * Dominique Van Der Straeten View author publications You can also search for
this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Dominique Van Der Straeten. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Chaerle, L., Caeneghem, W., Messens, E. _et al._ Presymptomatic visualization of plant–virus interactions by thermography . _Nat Biotechnol_ 17, 813–816 (1999). https://doi.org/10.1038/11765
Download citation * Received: 05 March 1999 * Accepted: 15 April 1999 * Issue Date: August 1999 * DOI: https://doi.org/10.1038/11765 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
