Exchange pathways of plastoquinone and plastoquinol in the photosystem ii complex
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Plastoquinone (PLQ) acts as an electron carrier between photosystem II (PSII) and the cytochrome b6f complex. To understand how PLQ enters and leaves PSII, here we show results of
coarse grained molecular dynamics simulations of PSII embedded in the thylakoid membrane, covering a total simulation time of more than 0.5 ms. The long time scale allows the observation of
many spontaneous entries of PLQ into PSII, and the unbinding of plastoquinol (PLQol) from the complex. In addition to the two known channels, we observe a third channel for PLQ/PLQol
diffusion between the thylakoid membrane and the PLQ binding sites. Our simulations point to a promiscuous diffusion mechanism in which all three channels function as entry and exit
channels. The exchange cavity serves as a PLQ reservoir. Our simulations provide a direct view on the exchange of electron carriers, a key step of the photosynthesis machinery. SIMILAR
CONTENT BEING VIEWED BY OTHERS ALGAL PHOTOSYSTEM I DIMER AND HIGH-RESOLUTION MODEL OF PSI-PLASTOCYANIN COMPLEX Article Open access 13 October 2022 FUNCTIONAL BASIS OF ELECTRON TRANSPORT
WITHIN PHOTOSYNTHETIC COMPLEX I Article Open access 10 September 2021 CRYO-EM AND FEMTOSECOND SPECTROSCOPIC STUDIES PROVIDE MECHANISTIC INSIGHT INTO THE ENERGY TRANSFER IN
CPCL-PHYCOBILISOMES Article Open access 05 July 2023 INTRODUCTION Photosynthetic organisms convert light into chemical energy. This fundamental process involves four major protein complexes:
photosystem II (PSII), cytochrome b6f complex (Cyt b6f), photosystem I and ATP synthase1,2. The process starts at PSII, which extracts electrons from water. The electrons travel
subsequently to Cyt b6f and PSI, after which they reduce NADP+. The electrons are transported between these protein complexes by charge carriers. Plastoquinone (PLQ) is the charge carrier
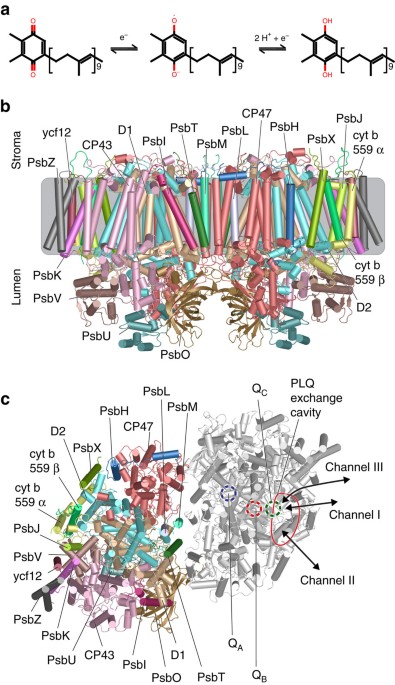
responsible for the electron transport from PSII to Cyt b6f. Upon photoactivation of PSII, PLQ is double reduced and takes up two protons to become plastoquinol (PLQol) (Fig. 1a). The PSII
core complex, a homodimer consisting of 27 subunits in plants and 20 in cyanobacteria3,4,5, coordinates two PLQs per monomer, named QA and QB, symmetrically positioned around a non-heme iron
(Fig. 1b,c, ). QA is stationary and does not leave the protein; it just passes the electron on to QB. QB, however, leaves the protein after its conversion to PLQol; then a new QB enters the
binding site and the process can start again. In the X-ray structure of Guskov _et al_.3 a third PLQ is present, coined QC. QC is however not found in the later Umena and Wei structures4,5.
The QC site is located close to the QB site, but the role of QC is still highly debated. While the QA site is well buried in the core of the PSII complex, the QB and QC sites connect to a
small cavity located within the protein (Fig. 1c). This so-called PLQ/PLQol exchange cavity is filled with lipids: on the stromal side with the negative charged phosphatidylglycerol (PG) and
sulfoquinovosyldiacylglycerol (SQDG) lipids and on the lumenal side with digalactosyldiacylglycerol (DGDG) and monogalactosyldiacylglycerol (MGDG) lipids4. Two channels link the cavity to
the thylakoid membrane3. Channel I, containing the tail of QC, is flanked by cyt b559α and PsbJ and opens up to the centre of the thylakoid membrane. The QC head group protrudes into the PLQ
exchange cavity, where it interacts with lipid and cofactor tails, but not with any amino acids. Channel II, containing the QB tail, is located between the D2 subunit and cyt b559β, opening
up more on the stromal side compared to channel I. The QB headgroup is located close to the non-heme iron. The presence of these two channels led to three different models for PLQ/PLQol
diffusion involving the QB and QC sites3. In the ‘alternating’ mechanism, channels I and II are used both as an entry and as an exit. Each PLQol leaves however through the same channel as
through which it entered as a PLQ. In the ‘wriggling’ mechanism, PLQ enters via channel I and PLQol leaves via channel II. This would be in line with the fact that PLQol is more polar than
PLQ, preferring to leave through channel II which opens up closer to the membrane surface6,7. In the ‘single channel’ mechanism, only channel II is used and channel I is occupied by a
stationary PLQ molecule (QC) that might be involved in redox reactions with cyt b559 (refs 8, 9). Here we present the results of coarse grained (CG) molecular dynamics (MD) simulations of
the diffusion of PLQ and PLQol in and out of the cyanobacterial PSII complex. The use of a CG model enables simulations of multi-meric protein assemblies in a complex membrane environment,
exploring time scales in the microsecond range10,11,12,13,14. Recently, we described the dynamics of the PSII complex including all of its cofactors, embedded in a realistic description of
the thylakoid membrane based on the CG Martini model15. We extended this work, and now focus our analysis on the exchange pathways of PLQ and PLQol by quantifying the role of the different
channels. Previous computational work has focused on the energetics of the QA and QB binding sites16, and on simulation of PLQ either in solution16 or in the thylakoid membrane17; for a
review see ref. 18. An atomistic simulation has recently been performed on the difference in binding of PLQ/PLQol at the QB site19, but the simulation time was too short to observe any
unbinding events. To our knowledge no simulations have been performed to address the full exchange of PLQ/PLQol in and out of PSII, which is the topic of the current manuscript. We simulated
the PSII dimer complex of the cyanobacteria _T. vulcanus_, including all cofactors and embedded in a realistic representation of the thylakoid membrane composed of MGDG, DGDG, SQDG and PG
lipids (Fig. 2). To probe the exit and entry pathways of PLQ and PLQol, the QB site was initially occupied by a PLQol, and PLQs were placed in the bulk membrane at about 5 mol%. Five
replicate simulations were performed for a time period between 80 and 100 μs each (see Methods for details). We observe multiple binding and unbinding events of the electron carriers, and
discover a new pathway, denoted channel III (Fig. 1c), by which PLQ and PLQol can enter or leave the PSII complex. RESULTS PLQOL LEAVES THE QB SITE ON A MICROSECOND TIME SCALE In our
previous simulations of the PSII dimer we showed that the oxidized form of the electron carrier, PLQ, remains stably bound at the QB site15. We anticipate that the reduced form, PLQol,
should leave the binding site in order to deliver two electrons to the cytochrome b6f complex. In line with our expectations, we observe the spontaneous unbinding of PLQol from the QB site.
Out of ten PLQols (two per dimer), five of the PLQols leave the QB site completely during the simulation. Out of these five, one PLQol escapes the protein ending up in the bulk thylakoid
membrane, two end up in the PLQ exchange cavity, and two remain trapped in the channels. The other five PLQols stay largely in place, with only transient or partial unbinding events being
observed (for example, rapid rebinding, or unbinding of the head group but not of the tail). Individual unbinding times vary greatly, from within 0.1 to tens of microseconds, pointing out
the stochastic nature of the unbinding process (see Supplementary Fig. 1). Note time scales in CG MD are only approximate; please see the SI for a more detailed discussion on their
interpretation. PLQ ENTERS EXCHANGE CAVITY AND REACHES BINDING SITES To investigate how PLQs diffuse into the PLQ exchange cavity, we calculated a PLQ density map, combining the data from
the five replicate simulations that contained additional PLQ molecules (138 molecules, ∼5 mol%) in the surrounding bulk thylakoid membrane. The resulting density map is visualized in Fig.
3a, showing the areas around the PSII dimer where it is more likely to encounter a PLQ during the simulation. The first remarkable feature is the non-homogeneous distribution of PLQ around
the protein. PLQ clearly has a preferred region of interacting with PSII, ranging approximately from subunits PsbZ to PsbH, with a few spots around CP43 and D1 where the PLQ tries to
penetrate PSII. Remarkably, there is no density in and around the dimer cleft. It appears that PLQs accumulate at the side of the protein with access to the exchange cavity. The exchange
cavity itself is also clearly visible in the density map. This implies that PLQs spontaneously enter the exchange cavity from the bulk thylakoid membrane. Interestingly the density is not
homogenously distributed within the cavity, but located more towards the cyt b559 and away from CP43 (Fig. 3a). At the end of the simulation the amount of PLQ inside the exchange cavity is
1.0±.2 (s.e.m., _n_=10) PLQ molecules. It is likely that this is still an underestimation, as equilibration of the PLQ population in the exchange cavity is a slow process. Note that, in most
simulations, a PLQol molecule is also present, either at the QB site or trapped in the exchange cavity. Despite the presence of PLQ in the exchange cavity we did not observe in any of the
simulations PLQ docking into the QB site, which would in principle be possible in the cases in which PLQol has left. This is reflected by the lack of density right at the QB site. The QC
site, on the other hand, is frequently visited by PLQs as is apparent from the density map. In addition, the QA site, containing the stationary PLQ molecule, is clearly visible. Visual
inspection of the trajectories shows that subtle side chain reorientations, especially of D1-HIS215, that occur after unbinding of PLQol, prevent easy access to the QB binding site. In order
to increase the chance of a PLQ binding to the QB site, more than 50 μs of adaptive MD was performed (see Methods). During the adaptive MD simulations PLQ could closely approach the binding
position found in the crystal structure, although with a slightly different orientation of the PLQ headgroup (see Supplementary Fig. 2). DISCOVERY OF A THIRD EXCHANGE CHANNEL Interestingly,
the PLQ density map shows the existence of three clear distinct pathways connecting the membrane to the PLQ exchange cavity, indicated by arrows in Fig. 3a. These pathways correspond to
channels I and II previously reported in the literature3, as well as a novel pathway, denoted channel III. Our data thus imply the existence of a third exchange channel. The novel channel
emerges when PsbJ moves towards cyt b559α while PsbK and ycf12 move in the opposite direction, creating a tunnel between PsbJ on one side and PsbK and ycf12 on the other side. To get an
estimate about the PLQ fluxes through the three channels we counted how many PLQs pass through each channel in the five different simulations (see Supplementary Methods, Supplementary Table
1). The data are shown in Table 1, averaged over the ten monomers (results for the individual monomers are given in Supplementary Table 2). In total, we observed 19 full entries and 11 full
exits, over the 0.5 ms aggregate simulation time. In addition, many additional PLQs are found trapped inside the channels at the end of the simulation. Our data furthermore show that each of
the channels is used both as an entrance and as an exit to the PLQ cavity. Sometimes a different channel is used to leave the cavity than the channel used to enter. It is however also
common that the same channel is used. In most of the complexes, the entry flux is higher than the exit flux (Table 1), causing the net increase in the amount of PLQs present in the PLQ
exchange cavity (see above). On average, every 33 μs a cofactor enters or leaves one of the two PLQ cavities. Comparing the fluxes between the channels, it appears that channels I and III
are used by PLQ with roughly the same frequency, but significantly fewer cofactors pass through channel II. In the latter channel, they often get stuck and do not completely pass through the
channel (see also Supplementary Table 2), which we attribute to the smaller size of channel II. Channel I has dimensions of 1.0 × 2.0 nm2, while the dimensions of channel II are only 1.0 ×
1.2 nm2 (ref. 20). We estimate the size of channel III as 1.1 × 2.0 nm2, similar to channel I (see Supplementary Methods). During the simulation, the shape and size of the channels
fluctuates significantly, with sizes transiently increasing or decreasing up to 100%. To explore whether PLQols can also enter the cavity using the same channels, six additional simulations
were performed (aggregate time 0.25 ms) with PLQol at 5 mol% in the bulk membrane (see Supplementary Methods). The results (Supplementary Tables 3 and 4) indicate a very similar behaviour as
observed for PLQ, implying that PLQol is potentially able to (re)enter the PLQ exchange cavity. Visual inspection of our simulation trajectories reveals that the subunits lining the
channels are dynamic, and can move with respect to each other. In particular, there is movement of the helices cyt b559α+β, PsbJ, PsbK ycf12 and PsbX. These relative movements can either
result in constriction or even complete closure of a channel, or result in an increased capacity of a channel due to a larger channel opening. This is especially noticeable for channels I
and III. Graphical snapshots illustrating the entrance of PLQ through channel I, the exit of PLQol through channel III and the opening of channel III are shown in Fig. 3b,c. See also
Supplementary Movies 1–3. PLQS CAN REORIENT INSIDE THE EXCHANGE CAVITY Also of interest is the observation that both PLQs and PLQols can enter the channels in two orientations, headgroup
first or tail first (see Supplementary Fig. 3). Inside the exchange cavity, PLQ can further reorient by flip-flopping between the stromal and lumenal leaflets. We observed 14 such events
during the 0.5 ms aggregate time of the five replicate simulations. Taking into account the amount and time PLQ molecules spent inside the cavity, this corresponds to a flip-flop time scale
of the order of 100 μs. Compared to the typical flip-flop time of PLQ in the thylakoid bulk membrane, which is around 1 μs as estimated from simulations using the same CG model17,
reorientation dynamics inside the cavity is thus slowed down by two orders of magnitude. In case of PLQol, no flip-flop events were observed for the PLQol molecules inside the cavity, in any
of the six replicate simulations. Again taking into account the amount and time spent in the cavity, we estimate a lower bound of the reorientation time scale of 200 μs. The lower flip-flop
rate of PLQol compared to PLQ is in line with the lower flip-flop rate for PLQol versus PLQ in bulk membranes (at least one order difference17,21), and can be attributed to the more polar
nature of the reduced cofactor. DISCUSSION We have investigated the behaviour of PLQ and PLQol in PSII, based on coarse-grained molecular dynamics simulations. The CG approach allowed us to
simulate the full PSII dimer system, including all cofactors and embedded in a realistic thylakoid membrane environment, on an aggregate time scale of almost 0.5 ms. We find that PLQs
accumulate around the PSII complex at sides close to the exchange cavity, and are able to enter and leave this cavity using three different channels. Of these, two channels correspond to the
known channels I and II. The third channel is a novel channel that has not been reported before. Taken together, our simulations point to a plastic behaviour of the PLQ exchange channels.
Here we discuss our results in light of the current literature view on PLQ binding and exchange pathways. A discussion of the limitations of our approach can be found in the Supporting
Information (Supplementary Note 1). In light of these limitations, it is imperative that our results are eventually verified by more detailed all-atom models, and/or validated
experimentally. In our simulations with PLQ occupying the QB site, both QA and QB remain stationary. This is in line with their function in photosynthesis. Experiments suggests that it is
relatively difficult to remove QA from its binding site22,23,24. The QB PLQ is only expected to leave the site after being converted to PLQol. This is confirmed by our simulations: we
observe that while all PLQs remain in the QB pocket, PLQols diffuse out of this binding site. The affinity of PLQ and PLQols for the QC site appears weak. Although the site is frequently
visited by PLQ/PLQols diffusing through channel I, actual binding is not observed. We hypothesize that the QC site in the crystal structure of Guskov _et al_.3 either originates from a PLQ
trapped inside the channel under the crystallization conditions, or represents a number of weaker binding spots around the QC site. The simulations reveal that there are preferential regions
on PSII where PLQ interacts, and from which PLQ can enter the exchange cavity. Importantly, our data suggest that the PLQ cavity could function as a PLQ reservoir. We quantified a gain of
about one PLQ molecule per monomer compared with the Umena structure in which only QB is present4. Considering our simulations did not reach equilibrium yet, the equilibrium population could
be even higher. The PLQ exchange cavity can thus function as a kind of local reservoir of PLQs, where PLQs can reorient. The latter is especially relevant because some of the PLQs enter
tail first. Note that the PLQ cavity reservoir should not be confused with the total PLQ pool, which is likely located for a large part outside the PSII complex in the thylakoid membrane,
and estimated to contain between 9 and 30 PLQs per complex25,26. The entry and exit kinetics of PLQ, on average one PLQ per 33 μs per monomer, is fast compared to both the first reduction
step of PLQ at the QB site towards semiplastoquinone, that has a time constant of a few hundreds of microseconds, and the second redox step that shows somewhat slower kinetics26,27. This
implies that a PLQ is always available close to the QB site to replace PLQ upon its reduction to PLQol, at least when the PLQ pool is largely oxidized. Interestingly, the simulations with
excess PLQol in the thylakoid membrane showed that the reduced electron carrier is able to potentially re-enter the exchange cavity, competing with PLQ. This could be an important regulation
mechanism to reduce reduction rates under conditions when PSII is operating too fast and the PLQ pool becomes reduced. Our simulations provide direct evidence for the existence of
designated PLQ/PLQol exchange pathways. Surprisingly, we find exchange taking place via three distinct channels, rather than two channels that are thus far assumed. Apart from channel I and
channel II that have been described before3, we observed a third channel between subunits PsbJ and ycf12/PsbK. The simulations show that channels I and III are used more or less equally, but
that the narrower channel II is used significantly less. The subunits lining the three channels can undergo conformational changes, modulating the relative opening or closure of channels I
and III in particular. Is channel III really used _in vivo_? Mutants studies have already shown that PsbJ is likely to be involved in the electron transport from QA to the PLQ pool, pointing
to the importance of cyt b559 for photosynthesis, and revealed that PsbX has an influence on PLQ turnover28,29,30,31,32,33,34. However, a way to really assess the existence of channel III
_in vitro_ could be by measuring the distance between the various helices over time. This could be achieved by the inclusion of fluorescent or electromagnetic probes in helices cyt b559α,
PsbJ and ycf12 and subsequently reading out the distances between the subunits over time using FRET or EPR measurements. Closing channels, by crosslinking the constituent helices, might be
another approach to study the existence and the usage of the various channels. One might be able to close, for example, channel III by crosslinking PsbJ, ycf12 and PsbK together and
subsequently measure the effect on the redox potential of QA and the PLQ pool. In the literature, three different PLQ exchange mechanisms have been proposed: the alternating, the wriggling
and the single channel mechanism3. The simulations do not match any of them fully. Our data agree with the alternating mechanism in the sense that both channels are used as an entry and an
exit. It differs to the point that the cofactors do not have to leave through the same channel as through which they entered. Our simulations agree with the wriggling mechanism to the point
that channel I can be used for PLQ entry and channel II for PLQol exit. In the wriggling model, however, channel I is exclusively used as an entry and channel II exclusively as an exit. We
observe that PLQs enter the PLQ cavity using all channels and that PLQol can leave the binding site also making use of all three channels. Our simulations do not agree with the single
channel mechanism in which only channel II is actively used and channel I is occupied by a stationary PLQ. In our simulations, the flux through channel I is actually significantly larger.
Our findings are therefore also in disagreement with the conclusions derived from the MD study of Zobnina _et al_.19, apparently supporting the existence of a single channel model. Our
simulations are however four orders of magnitude longer and Zobnina _et al_.19 do not observe any actual exchange of PLQs or PLQols. Finally, in all three mechanisms the PLQs directly
diffuse to the QB site after entering a channel and directly leave the complex after being reduced to PLQol. In contrast, we observe the exchange cavity to serve as a PLQ reservoir allowing
for substantial PLQ rearrangements before/after binding/unbinding. The model emerging from our simulations is one in which three channels exist, each serving as both entry and exit pathway
for both PLQ and PLQol. The exchange cavity serves as a temporary PLQ reservoir in which the PLQs can reorient. We denote this model the ‘promiscuous’ mechanism, to be considered as
alternative for the alternating, the wriggling and the single channel mechanisms. A schematic of the new model is shown in Fig. 4. Taken together, based on large-scale simulations, we have
been able to shed important light on the mechanism by which PLQs and PLQols can diffuse in and out of the PSII complex. Our simulations do not fully agree with any of the three diffusion
mechanisms described in the literature3. Instead they point to a less organized, less deterministic model. Nine main observations can be made. (1) Three different channels exist that all can
be used as an entry and an exit channel. (2) The entry and exit channel do not have to be the same for an individual PLQ; a number of PLQs enter and leave through the same channel, but
others do this by a different channel. (3) The QC site likely represents a weaker binding spot. (4) PLQs can pass through the channels in at least two different orientations, with their
headgroup first or with their tail first. (5) PLQs do not directly dock at the QB site from the channel, instead they first enter in the PLQ exchange cavity where they can diffuse around and
reorient themselves. (6) PLQs can accumulate in the PLQ exchange cavity forming a PLQ reservoir. (7) The flux through channels I and III is more or less equal and several times larger than
through channel II. (8) The relative flux through channels I and III is influenced by the relative conformations of cyt b559, PsbJ, ycf12 and PsbK. Possibly PsbX might be able to influence
the flux through channel II. (9) The side of the protein where the channel openings are located acts as a funnel, accumulating PLQs towards the entrances of the exchange cavity. The
combination of these nine observations leads to a new model, the ‘promiscuous’ mechanism, in which channels I and III are primarily used, and each channel functions as an uncorrelated entry
and exit of PLQ/PLQol. The PLQ exchange cavity can function as a local PLQ supply in which the PLQs and PLQols can reorient and there is a regulatory function of subunits cyt b559, PsbJ,
ycf12 and PsbK. METHODS SYSTEM SETUP To study the dynamics of PLQ and PLQol exchange in the PSII complex, we used the equilibrated structure from our previous simulations of the PSII
complex15. Here, we describe five independent simulations of the dimer in which the QB pocket contained a PLQol and extra PLQs were added to the thylakoid membrane. The simulations are based
on the crystal structure of the cyanobacterial PSII complex from Umena _et al_.4 with PDB ID: 3ARC. The protein was coarse grained together with all of its cofactors and embedded in a
thylakoid membrane composed out of 2,686 lipids using the _insane_ script35. The membrane is composed of the negative charged PG and SQDG, and the neutral MGDG and DGDG lipids with oleoyl
and palmitoyl tails, a realistic representation of the thylakoid membrane17,36. The system further contains 73,144 CG water beads (representing four times as many water molecules), 455 Na+
and 455 Cl− ions (around 100 mM NaCl), plus 1032 Na+ counter ions to neutralize the overall charge. More details of the setup of this system can be found in our recent publication15. The
five different simulations described in the current work all started from the same initial structure, but with different seeds for the initial randomized velocities. In order to investigate
the pathways of PLQ diffusion to the QB binding site, 69 PLQ molecules were inserted into each membrane leaflet at the start of the simulation, totalling to 138 free PLQs, yielding a
concentration of about 5 mol % in the membrane. The PLQs were added to a pre-equilibrated bilayer by increasing the lateral dimensions of the box by 1.27 nm and adding 69 PLQs to each
leaflet using the _insane_ script35. The PLQs present in the QB sites were modified to PLQols. The QC site was left unoccupied. SIMULATION DETAILS The Martini force field version 2.2 (refs
37, 38) in conjunction with the ElNeDyn elastic network39 were used to model the interactions. The PLQ and PLQol parameters originate from ref. 21. The lipid parameters were taken from ref.
40 with the modification as described in ref. 17. GROMACS version 4.5.5 (ref. 41) was used to integrate the equations of motion with the common Martini settings for the Martini force
field42. The simulations were run in the isothermal-isobaric (NpT) ensemble. Since _T. vulcanus_ is a thermophile, the simulations were performed at 328 K, maintaining the thylakoid membrane
in the fluid phase. The temperature was controlled using the V-rescale thermostat with a coupling constant of _τ_t=2.0 ps (ref. 43). The pressure was semi-isotropically coupled to an
external bath of _p_=1 bar with a coupling constant of _τ_p=1.0 ps and a compressibility of _χ_=3.0−4 bar−1 using the Berendsen barostat44. A shifted potential with a cutoff of 1.2 nm in
conjunction with a dielectric constant of 15 was used to model the electrostatic interactions. The Van der Waals interactions were also calculated using a shifted potential, with a cut off
of 1.2 nm and a switch at 0.9 nm. The five replicate simulations had a length of 84.2, 92.1, 97.4, 94.3 and 106.5 μs, summing up to almost 0.5 ms. Trajectories were saved and analysed every
500 ps, the first 1 μs being discarded as equilibration time. In order to increase the chance of a PLQ docking to the QB site, an adaptive MD simulation approach was used consisting of an
iterative process in which new, relative short simulations are spawn from promising configurations. Such an adaptive approach has been shown to be efficient in observing rare events, such as
the binding of a ligand to its binding pocket45. Here, configurations were selected in which a PLQ was in a favourable condition to approach the bicarbonate ion. All together, we performed
41 independent short simulations totalling more than 50 μs simulation time. Details of the analysis, and additional simulations with excess PLQol, can be found in the Supporting Information
(Supplementary Methods)46,47. Visual molecular dynamics (VMD) (ref. 46) and pymol (ref. 47) were used to generate the images. DATA AVAILABILITY The authors declare that all data supporting
the findings of this study are available within the manuscript and its supplementary files or are available from the corresponding author on request. ADDITIONAL INFORMATION HOW TO CITE THIS
ARTICLE: Van Eerden, F. J. _et al_. Exchange pathways of plastoquinone and plastoquinol in the photosystem II complex. _Nat. Commun._ 8, 15214 doi: 10.1038/ncomms15214 (2017). PUBLISHER’S
NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. REFERENCES * Blankenship, R. E. _Molecular Mechanisms of
Photosynthesis_ Wiley Blackwell (2014). * Croce, R. & van Amerongen, H. Natural strategies for photosynthetic light harvesting. _Nat. Chem. Biol._ 10, 492–501 (2014). Article CAS
Google Scholar * Guskov, A. et al. Cyanobacterial photosystem II at 2.9-Å resolution and the role of quinones, lipids, channels and chloride. _Nat. Struct. Mol. Biol._ 16, 334–342 (2009).
Article CAS Google Scholar * Umena, Y., Kawakami, K., Shen, J.-R. & Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9Å. _Nature_ 473, 55–60 (2011).
Article CAS ADS Google Scholar * Wei, X. et al. Structure of spinach photosystem II–LHCII supercomplex at 3.2 Å resolution. _Nature_ 534, 69–74 (2016). Article CAS ADS Google Scholar
* Nowicka, B. & Kruk, J. Occurrence, biosynthesis and function of isoprenoid quinones. _BBA—Bioenergetics_ 1797, 1587–1605 (2010). Article CAS Google Scholar * Müh, F., Glöckner,
C., Hellmich, J. & Zouni, A. Light-induced quinone reduction in photosystem II. _Biochim. Biophys. Acta_ 1817, 44–65 (2012). Article Google Scholar * Bondarava, N. et al. Evidence that
cytochrome b559 mediates the oxidation of reduced plastoquinone in the dark. _J. Biol. Chem._ 278, 13554–13560 (2003). Article CAS Google Scholar * Kaminskaya, O., Shuvalov, V. A. &
Renger, G. Evidence for a novel quinone-binding site in the photosystem II (PS II) complex that regulates the redox potential of cytochrome b559. _Biochemistry_ 46, 1091–1105 (2007). Article
CAS Google Scholar * Ingólfsson, H. I. et al. Lipid organization of the plasma membrane. _J. Am. Chem. Soc._ 136, 14554–14559 (2014). Article Google Scholar * Arnarez, C., Marrink, S.
J. & Periole, X. Molecular mechanism of cardiolipin-mediated assembly of respiratory chain supercomplexes. _Chem. Sci._ 7, 4435–4443 (2016). Article CAS Google Scholar * Chavent, M.,
Duncan, A. L. & Sansom, M. S. Molecular dynamics simulations of membrane proteins and their interactions: from nanoscale to mesoscale. _Curr. Opin. Struct. Biol._ 40, 8–16 (2016).
Article CAS Google Scholar * Koldsø, H. & Sansom, M. S. P. Organization and dynamics of receptor proteins in a plasma membrane. _J. Am. Chem. Soc._ 137, 14694–14704 (2015). Article
Google Scholar * Holdbrook, D. A., Huber, R. G., Piggot, T. J., Bond, P. J. & Khalid, S. Dynamics of crowded vesicles: local and global responses to membrane composition. _PLoS ONE_ 11,
e0156963 (2016). Article Google Scholar * van Eerden, F. J. et al. Molecular dynamics of photosystem II embedded in the thylakoid membrane. _J. Phys. Chem. B_ 121, 3237–3249 (2017).
Article CAS Google Scholar * Ishikita, H., Hasegawa, K. & Noguchi, T. How does the QB site influence propagate to the QA site in photosystem II? _Biochemistry_ 50, 5436–5442 (2011).
Article CAS Google Scholar * van Eerden, F. J., de Jong, D. H., de Vries, A. H., Wassenaar, T. A. & Marrink, S. J. Characterization of thylakoid lipid membranes from cyanobacteria and
higher plants by molecular dynamics simulations. _BBA—Biomembranes_ 1848, 1319–1330 (2015). Article CAS Google Scholar * Lambreva, M. D. et al. Structure/function/dynamics of photosystem
II plastoquinone binding sites. _Curr. Protein Pept. Sci._ 15, 285–295 (2014). Article CAS Google Scholar * Zobnina, V. et al. The plastoquinol-plastoquinone exchange mechanism in
photosystem II: insight from molecular dynamics simulations. _Photosynth. Res._ 131, 15–30 (2017). Article CAS Google Scholar * Kern, J., Zouni, A., Guskov, A. & Krauss, N. in _Lipids
in Photosynthesis_ Vol. 30 (eds Wada, H. & Murata, N.) 203–242Springer Netherlands (2009). * de Jong, D. H. et al. Atomistic and coarse grain topologies for the cofactors associated
with the photosystem II core complex. _J. Phys. Chem. B_ 119, 7791–7803 (2015). Article CAS Google Scholar * Ermakova-Gerdes, S. & Vermaas, W. Mobility of the primary
electron-accepting plastoquinone QA of photosystem II in a _Synechocystis_ sp. PCC 6803 strain carrying mutations in the D2 protein. _Biochemistry_ 37, 11569–11578 (1998). Article CAS
Google Scholar * Diner, B. A., de Vitry, C. & Popot, J.-L. Quinone exchange in the QA binding site of Photosystem II reaction center core preparations isolated from _Chlamydomonas
reinhardtii_. _BBA—Bioenergetics_ 934, 47–54 (1988). Article CAS Google Scholar * Araga, C. et al. Functional reconstitution of the primary quinone acceptor, QA, in the Photosystem II
core complexes. _BBA—Bioenergetics_ 1142, 36–42 (1993). Article CAS Google Scholar * Govindjee, G. _Chlorophyll a fluorescence: a bit of basics and history_ Kluwer Academic Publishers
(2004). * Kolber, Z. & Falkowski, P. G. Use of active fluorescence to estimate phytoplankton photosynthesis _in situ_. _Limnol. Oceanogr._ 38, 1646–1665 (1993). Article CAS ADS Google
Scholar * Kern, J. & Renger, G. Photosystem II: Structure and mechanism of the water:plastoquinone oxidoreductase. _Photosynth. Res._ 94, 183–202 (2007). Article CAS Google Scholar
* Katoh, H. & Ikeuchi, M. Targeted disruption of psbX and biochemical characterization of photosystem II complex in the thermophilic cyanobacterium _Synechococcus elongatus_. _Plant Cell
Physiol._ 42, 179–188 (2001). Article CAS Google Scholar * Lind, L. K., Shukla, V. K., Nyhus, K. J. & Pakrasi, H. B. Genetic and immunological analyses of the cyanobacterium
_Synechocystis_ sp. PCC 6803 show that the protein encoded by the _psbJ_ gene regulates the number of photosystem II centers in thylakoid membranes. _J. Biol. Chem._ 268, 1575–1579 (1993).
CAS PubMed Google Scholar * Nowaczyk, M. M. et al. Deletion of psbJ leads to accumulation of Psb27–Psb28 photosystem II complexes in _Thermosynechococcus elongatus_. _BBA—Bioenergetics_
1817, 1339–1345 (2012). Article CAS Google Scholar * Ohad, I., Dal Bosco, C., Herrmann, R. G. & Meurer, J. Photosystem II proteins PsbL and PsbJ regulate electron flow to the
plastoquinone pool. _Biochemistry_ 43, 2297–2308 (2004). Article CAS Google Scholar * Pakrasi, H. B., Williams, J. G. & Arntzen, C. J. Targeted mutagenesis of the _psbE_ and _psbF_
genes blocks photosynthetic electron transport: evidence for a functional role of cytochrome b559 in photosystem II. _EMBO J._ 7, 325 (1988). Article CAS Google Scholar * Regel, R. E. et
al. Deregulation of electron flow within photosystem II in the absence of the PsbJ protein. _J. Biol. Chem._ 276, 41473–41478 (2001). Article CAS Google Scholar * Shinopoulos, K. E. &
Brudvig, G. W. Cytochrome b559 and cyclic electron transfer within photosystem II. _BBA—Bioenergetics_ 1817, 66–75 (2012). Article CAS Google Scholar * Wassenaar, T. A., Ingólfsson, H.
I., Böckmann, R. A., Tieleman, D. P. & Marrink, S. J. Computational lipidomics with insane: a versatile tool for generating custom membranes for molecular simulations. _J. Chem. Theory
Comput._ 11, 2144–2155 (2015). Article CAS Google Scholar * Sakurai, I. et al. Lipids in oxygen-evolving photosystem II complexes of cyanobacteria and higher plants. _J. Biochem._ 140,
201–209 (2006). Article CAS Google Scholar * de Jong, D. H. et al. Improved parameters for the martini coarse-grained protein force field. _J. Chem. Theory Comput._ 9, 687–697 (2013).
Article CAS Google Scholar * Marrink, S. J., Risselada, H. J., Yefimov, S., Tieleman, D. P. & de Vries, A. H. The MARTINI force field: coarse grained model for biomolecular
simulations. _J. Phys. Chem. B_ 111, 7812–7824 (2007). Article CAS Google Scholar * Periole, X., Cavalli, M., Marrink, S.-J. & Ceruso, M. A. Combining an elastic network with a
coarse-grained molecular force field: structure, dynamics, and intermolecular recognition. _J. Chem. Theory Comput._ 5, 2531–2543 (2009). Article CAS Google Scholar * López, C. A.,
Sovova, Z., van Eerden, F. J., de Vries, A. H. & Marrink, S. J. Martini force field parameters for glycolipids. _J. Chem. Theory Comput._ 9, 1694–1708 (2013). Article Google Scholar *
Hess, B., Kutzner, C., van der Spoel, D. & Lindahl, E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. _J. Chem. Theory Comput._ 4, 435–447
(2008). Article CAS Google Scholar * de Jong, D. H., Baoukina, S. & Ingólfsson, H. I. Martini straight: boosting performance using a shorter cutoff and GPUs. _Comp. Phys. Commn._ 199,
1–7 (2016). Article CAS ADS MathSciNet Google Scholar * Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. _J. Chem. Phys._ 126, 014101 (2007).
Article ADS Google Scholar * Berendsen, H., Postma, J., van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. _J. Chem. Phys._ 81,
3684–3690 (1984). Article CAS ADS Google Scholar * Ozer, G., Keyes, T., Quirk, S. & Hernandez, R. Multiple branched adaptive steered molecular dynamics. _J. Chem. Phys._ 141, 064101
(2014). Article ADS Google Scholar * Humphrey, W., Dalke, A. & Schulten, K. VMD visual molecular dynamics. _J. Molec. Graph._ 14, 33–38 (1996). Article CAS Google Scholar *
Schrödinger, L. L. C. The PyMOL Molecular Graphics System, Version 1.7.0.0. (2010). Download references ACKNOWLEDGEMENTS We thank Albert Guskov for helpful discussions and critical reading
of the manuscript, and Marina Guskova for help with the illustrations. F.J.V.E. acknowledges funding from the Foundation of Fundamental Research of Matter (FOM), and S.J.M. acknowledges
funding through an ERC Advanced Grant ‘COMP-MICR-CROW-MEM’. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Groningen Biomolecular Sciences and Biotechnology Institute & Zernike Institute
for Advanced Materials, University of Groningen, Nijenborgh 4, Groningen, 9747 AG, The Netherlands Floris J. Van Eerden, Manuel N. Melo, Pim W. J. M. Frederix, Xavier Periole & Siewert
J. Marrink * Instituto de Tecnologia Química e Biológica António Xavier, Universidade Nova de Lisboa, Av. da República, Oeiras, 2780-157, Portugal Manuel N. Melo Authors * Floris J. Van
Eerden View author publications You can also search for this author inPubMed Google Scholar * Manuel N. Melo View author publications You can also search for this author inPubMed Google
Scholar * Pim W. J. M. Frederix View author publications You can also search for this author inPubMed Google Scholar * Xavier Periole View author publications You can also search for this
author inPubMed Google Scholar * Siewert J. Marrink View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS F.J.V.E., X.P. and S.J.M. designed the
research; F.J.V.E. and M.N.M. performed the research; all authors analysed the data and wrote the paper. CORRESPONDING AUTHOR Correspondence to Siewert J. Marrink. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figures, Supplementary Tables, Supplementary Note,
Supplementary Methods and Supplementary References (PDF 3302 kb) SUPPLEMENTARY MOVIE 1 Opening of Channel III. During the 3 μs movie Cyt b 559β, Cyt b 559α and PsbJ move and tilt in the
direction of subunit D2, while ycf12 and PsbK move in the opposite direction, resulting in the opening of channel III. Also visible is a PLQol occupying the QB site. The cofactor tail (red)
sticks out of channel II, while its headgroup (orange) is located at the QB site. View is from within the membrane, with the subunits colored as in Figure 1b. (MOV 23750 kb) SUPPLEMENTARY
MOVIE 2 Entering of PLQ inside the PLQ exchange cavity through Channel I. In this 35 μs movie a PLQ (blue, with headgroup in orange), enters the PLQ exchange cavity through channel I.
Interestingly the PLQ adapts a folded conformation while passing through the channel. After passing through the channel, the cofactor diffuses around in the cavity. View is stromally on the
protein, with the subunits colored as in Figure 1b. (MOV 21379 kb) SUPPLEMENTARY MOVIE 3 Leaving of PLQol through Channel III. The same PLQol (colored red, with its headgroup in orange) as
in Figure 3b diffuses during 22 μs from the QB site, with its tail in Channel II, via Channel III into the thylakoid membrane. View is stromally with an angle, with the subunits colored as
in Figure 1b. (MOV 18892 kb) PEER REVIEW FILE (PDF 310 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other
third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative
Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Van Eerden, F., Melo, M., Frederix, P. _et al._ Exchange pathways of plastoquinone and plastoquinol in the photosystem II
complex. _Nat Commun_ 8, 15214 (2017). https://doi.org/10.1038/ncomms15214 Download citation * Received: 11 November 2016 * Accepted: 01 March 2017 * Published: 10 May 2017 * DOI:
https://doi.org/10.1038/ncomms15214 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
