Water tribology on graphene | Nature Communications
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Classical experiments show that the force required to slide liquid drops on surfaces increases with the resting time of the drop, _t_rest, and reaches a plateau typically after
several minutes. Here we use the centrifugal adhesion balance to show that the lateral force required to slide a water drop on a graphene surface is practically invariant with _t_rest. In
addition, the drop’s three-phase contact line adopts a peculiar micrometric serrated form. These observations agree well with current theories that relate the time effect to deformation and
molecular re-orientation of the substrate surface. Such molecular re-orientation is non-existent on graphene, which is chemically homogenous. Hence, graphene appears to provide a unique
tribological surface test bed for a variety of liquid drop-surface interactions. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY
OTHERS NON-WETTING OF CONDENSATION-INDUCED DROPLETS ON SMOOTH MONOLAYER SUSPENDED GRAPHENE WITH CONTACT ANGLE APPROACHING 180 DEGREES Article Open access 17 October 2022 SYNERGISTIC EFFECTS
OF CONFINEMENT SIZE AND INTERFACE ON ANOMALOUS ULTRAFAST TRANSPORT IN NANOFLUIDICS Article Open access 16 April 2025 SPONTANEOUS CHARGING AFFECTS THE MOTION OF SLIDING DROPS Article Open
access 14 April 2022 INTRODUCTION We learn in basic physics courses that the frictional force is proportional to the load (the Amonton law1). Tribology teaches us that the frictional force
is proportional to the contact area, whereas the Amonton law is a special case for very rough surfaces in which the load is roughly proportional to the contact area2. Recently, it was shown
that for drops on surfaces, the frictional force can be inversely proportional to the load if, for example, we compare sessile and pendant drops3,4. This peculiar behaviour is a result of
enhanced intermolecular interactions at the three-phase contact line which dominates the frictional force for this system3,4. The Shanahan-de Gennes surface deformation at the three-phase
contact line5,6 re-orients the molecules resulting in a new distribution of functional groups between those on the surface and those buried deeper in the surface. This enhances the
solid–liquid interaction and, hence, the drop’s friction or lateral force3,4. A requirement for this behaviour is that the solid surface be chemically heterogeneous so as to allow different
distributions of chemical functional groups between those that are on the surface and those buried deeper in it. This effect is associated with a variety of phenomena including the marked
differences between chemisorbed and physisorbed monolayers7 and wetting transitions8 with implications ranging from plant growth9 through electrovariable optical components10 to nanofiber
membranes11, which are chemically heterogeneous. Graphene, however, manifests a chemically homogeneous solid surface with peculiar mechanical friction and wear properties12,13,14,15,16. In
this paper, we show that for water drops on graphene, the retention force for sessile and pendant drops is equal within the experimental scatter. In addition, for most systems, the
re-orientation process described above reaches a plateau typically after about 10–20 minutes17. Here we show that for graphene, there is no variation in the retention force for drop sliding
as a function of the time the drop rests on the surface suggesting an instantaneous re-orientation process for graphene surfaces. We conclude that the homogeneous and stable nature of
graphene excludes the possibility of time changes in the intermolecular interactions between the liquid and the solid surface. Therefore, the only position for the three-phase contact line
to pin on the surface is at the boundaries where graphene domains that nucleated at two adjacent nucleation sites meet. These boundaries form a complex micrometric tessellation whose shape
is irrespective of the contact line, and hence should reduce its smooth nature. Indeed, we see that for graphene, the three-phase contact line forms a serrated micrometric structure, which
differs from the smooth line on other surfaces. RESULTS CRITICAL ANGULAR VELOCITY FOR DROP MOTION A novel way of studying liquid–solid interactions is possible by measuring the combination
of centrifugal and gravitational forces that drive liquid drops on solid surfaces using the recently established centrifugal adhesion balance (CAB)4. With this device, it is possible to
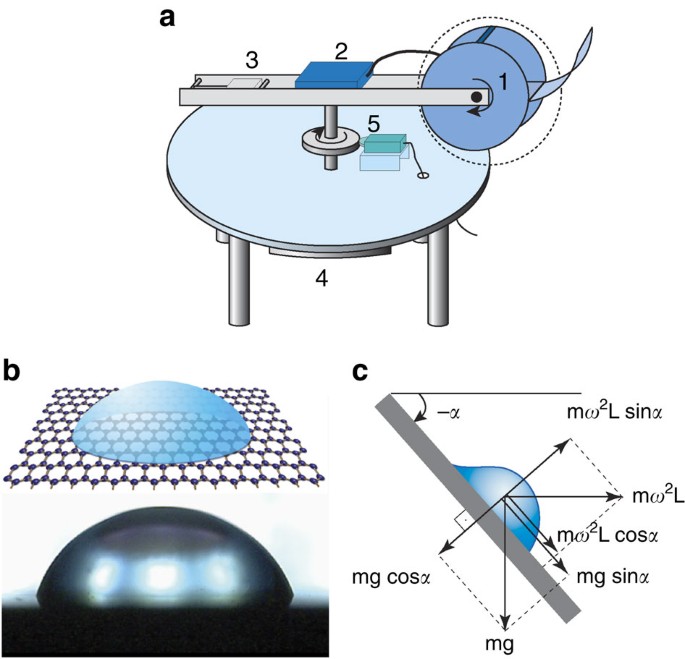
independently measure and manipulate normal (_f_┴) and lateral (_f_||) body forces acting upon the liquid drop. A schematic illustration of the CAB is shown in Fig. 1a. The setup consists of
a centrifugal arm on which a closed chamber is attached. The arm rotates horizontally using a computer-controlled dc motor. On one side of the arm is a counterbalance. On the other side is
the chamber plate, which can be fixed at any desired angle with respect to the axis of rotation. In addition, a charge-coupled device camera inside the CAB chamber records video signals of
the system in real time4. Figure 1b shows a picture of a 10-μl water drop on a graphene surface (see characterization in Supplementary Fig. S1 (Raman spectra) and S2 (transmission electron
microscopy (TEM))). Figure 2 shows how _ω_, the angular velocity of the CAB, is varied during a single measurement. Before rotating the CAB, different intervals of still time, _t_still, the
time the drop is resting undisturbed in the motionless CAB chamber, (time period (i)–(ii) in Fig. 2) are maintained. Following _t_still, _ω_ is gradually increased up to a critical value,
_ω_C ((ii)–(iii) in Fig. 2) at which the drop starts sliding along the surface. Before reaching _ω_C, the drop is pinned to the surface but is slowly deformed in shape as the angular
acceleration is increased. The time the drop is placed on the surface is time ‘zero’ in Fig. 2. The resting time, _t_rest, is the time from which the drop is placed on the surface to when
_ω_C is reached. RETENTION FORCE FOR GRAPHENE The normal and the lateral retention forces at _ω_C, (when the drop starts moving) are calculated (see Fig. 1c) as follows: where _m_ is the
mass of the drop, _g_ the gravitational acceleration, _L_ is the distance of the drop from the axis of rotation and _α_ the angle at which the CAB chamber plate is tilted with respect to the
arm axis. A 0° tilt corresponds to a true sessile (+1 g) configuration and a 180° tilt corresponds to a true pendant (−1 g) configuration. Recent studies4,17 show that the lateral force
required for sliding drops on surfaces increases with the resting time. A schematic representation of this time effect is plotted in Fig. 3a, showing _f_|| versus resting time. The force
increases with resting time, and after some period, _t_pl, which is usually about 10–20 min (a time which is typical for re-organization of solid surface molecules18,19,20), it reaches a
plateau, denoted by _t_rest=_t_pl as demonstrated in Fig. 3a. We refer to the _f_|| value that corresponds to _t_rest≥_t_pl as _f_||pl. The trend shown in Fig. 3a is valid for a variety of
drop-solid systems studied so far4,12,17,21,22,23. The effect of the resting time, _t_rest, on the lateral force, _f_||, required to set a water drop in motion on a graphene substrate is
very different. Figure 3b shows that _f_|| does not vary with _t_rest for water drops on graphene, regardless of the drop volume and the effective gravity the drop experiences, or the G/2D
values characteristic of the graphene layer. Thus, _f_||(_t_1)=_f_||(_t_2)=_f_||pl for graphene systems, where _f_||(_t_1) and _f_||(_t_2) represent _f_|| values of any experimentally
measurable resting times _t_1 and _t_2. Similarly, highly oriented pyrolytic graphite (HOPG), whose surface has a similar chemical nature to that of graphene, exhibits the same behaviour. In
addition to the time-independent behaviour of _f_|| displayed in Fig. 3b, note that the _f_||pl for sessile (+1 g) and pendant (−1 g) drops are similar for graphene surfaces (compare red
up-triangles with blue down-triangles in Fig. 3b) as opposed to the case of other known (and chemically heterogeneous), surfaces which exhibit significantly higher _f_||pl for pendant
drops4. DISCUSSION The classic increase of _f_|| as a function of the resting time shown in Fig. 3a has been theoretically related to the Shanahan-de Gennes deformation-induced
re-orientation of the solid surface molecules4,5,6,17, which enables stronger intermolecular interactions of the re-oriented solid molecules with the liquid (hence, higher _f_||). Such time
dependence of _f_|| is shown in Fig. 3a. For the graphene system shown in Fig. 3b, however, the general trend of Fig. 3a cannot be observed within the minimal time resolution of our
experiments (to avoid drop motion due to pulsation, the change in _ω__2__L_ needs to be gradual, leading to a minimal time of _t_active ~2 min to reach _ω_c sliding conditions). For an
atomically homogeneous surface, such as the sp2 carbon lattice in an ideal crystal of graphene (compare with Fig. 1b), the molecular re-orientation can only be expressed in the realignment
of the electronic orbitals, which is a phenomenon of the time order of attoseconds12. But if this was the mechanism associated with pinning the drop to the surface, then the slightest force
aiming to slide the drop should have allowed the liquid molecules at the three-phase contact line to induce orbitalic re-orientation on the solid molecules nearby, and hence the sliding of
the drop. In other words, there would be no force retaining the drop to the solid surface, although we know experimentally that this is not the case. Another mechanism to hold drops to a
surface is _via_ defects on the solid surface24. The liquid is either more energetically attracted to those defects compared with the rest of the surface or repelled by them. In either case,
this creates a serrated three-phase contact line, which is then pinned to the defects either by adhering to them or by adhering to the rest of the surface and avoiding the defects. The
defects on a graphene surface can emanate from the borders at the places where different domains of graphene that nucleated meet. Such junctions provide a morphologically different region
and a ‘defect’ for the water drop to pin on. Studies carried out for understanding evolution and morphology of the chemical vapour deposition-grown graphene sheets revealed explicit domain
structure25. Boundaries of the domains have mismatch in the atomic structure of the two meeting graphene layers, as well as Bernard stacking of the graphene layers as they overlap each
other26. Further imperfections on the size scale of the droplet (ca. 10–100 μm) are also expected, for example, ripples27 and point defects. The strength of the interaction with these
defects has no time dependence, and hence we see straight horizontal lines in Fig. 3b. Although the regions of defects are difficult to resolve, such serrated three-phase contact line can be
enhanced as the drop evaporates. Figure 4 shows that indeed the three-phase contact line of a graphene surface is serrated on a micron scale upon evaporation, whereas that of a non-graphene
surface is not. The lack of time dependence in the drop-retention force shown in Fig. 3b is therefore a manifestation of the lack of any molecular re-orientation that can lead to a stronger
solid–liquid intermolecular interaction of the liquid with the planar graphene surface. It should be noted that metals, being high-energy surfaces, have zero contact angle with almost any
liquid28. In general, most hard surfaces are also high-energy surfaces because breaking the bonds of a hard material requires high energy. Graphene is an exception: on one hand it is hard
and on the other hand, breaking the bonds between the layers, does not require as much energy. The deformation at the three-phase contact line that leads in other surfaces to molecular
re-orientation of the solid molecules can only lead to topographic deformation in graphene, but not molecular re-orientation. The two energetically different states of graphene, the part
inside the sheets and the part near their edges, have a chemical difference: the edges have dangling bonds, which are terminated by hydrogen atoms quickly after growth, whereas inside the
sheets are composed of the classic sp2 carbon lattice. All of these structures are already on the surface, and the mechanical strength and high-electrical conductivity of the graphene
coating prevent any other functional groups located deeper in the substrate to re-orient, such as to reach the surface. Therefore, we see this peculiar tribological behaviour for graphene
surfaces. The unique surface chemistry of graphene is also present in HOPG, and in Fig. 3b, we see that HOPG exhibits the same behaviour. This similarity supports attributing these
tribological observations to the surface chemistry rather than deeper layers of the substrate. In addition to the previous observation on graphene substrates regarding the time dependency of
_f_||, another interesting phenomenon observed in Fig. 3b is the similarity between _f_||pl for sessile (_a_┴=+1g) and pendant (_a_┴=–1g) drops. In contrast, for non-graphene surfaces, the
_f_||pl for pendant drops is distinctively higher than that for sessile drops4. In the case of surfaces that consist of chemically heterogeneous molecules (that is, molecules with different
chemical functional groups), the pinning force emanates from the enhanced intermolecular interaction associated with the deformation at the three-phase contact line. For pendant drops,
higher _f_ ||pl retention forces was attributed to gravity-induced facilitated three-phase contact-line deformation3,4,23. For the graphene sheet, however, the deformation of the surface
does not affect its intermolecular interactions with the liquid. Therefore, the forces associated with sliding pendant and sessile drops are similar. In summary, using the CAB, we show
experimentally that the retention force of water drops on graphene surfaces does not depend on the drop resting time, in contrast to any other known system. We further observe that the
forces required to slide sessile and pendant drops on graphene sheets are similar. Both of these tribological observations are attributed to the chemical homogeneity and stability of
graphene surfaces, which sustains the same morphology and chemical composition on the surface regardless of the Shanahan de-Gennes-type surface deformation. The retention force of the water
drops on graphene surface is therefore only attributed to the boundaries where domains of graphene that nucleated at two adjacent nucleation sites meet. This gives rise to a unique serrated
three-phase contact line on graphene surfaces. The three-phase contact line is pinned along the serrated structure of these domains and not to the Shanahan de-Gennes deformed ridge, and
because the domains were on the surface historically, there is no time dependence to the drop-retention force on graphene. METHODS MATERIALS Fabricated graphene based on the protocol of
Srivastava _et al_27 from Rice University was used. The distilled water used in the experiments was from Barnstead Nanopure Purification system, which provides specific conductance (at 25
°C) ≤0.7 × 10−6 Ω−1 cm−1. ParafilmM from Sigma-Aldrich was used as the goniometer sealing agent for all experiments performed using the CAB. HOPG grade SPI-1 (12 × 12 × 1 mm3) was obtained
from SPI Supplies and cleaved before use. We practiced slow cleaving of about 45 min for 1.4 cm2 of HOPG surface. The silicon wafers (diameter: 76.2±0.3 mm; orientation: <110>±0.9°;
resistivity: 0.0034–0.0046 Ω cm centre; thickness: 381±25 μm) used in this paper (obtained from Virginia Semiconductor) were cut into a 1.4 × 6-cm2 piece, rinsed with ethanol, then with
distilled water and dried in a StableTemp vacuum oven (model: 5053-10 from Cole-Parmer) at 100 °C for 30 min. Then it went through a 45 min cleaning using a ultraviolet/ozone Procleaner
(model: Procleaner 110) to remove any organic impurities on the surface. From the ultraviolet/ozone cleaner, the silicon was immediately placed in a 1% (volume) solution of
octadecyltrimethoxysilane (90% technical grade obtained from Sigma-Aldrich) in toluene (99.5% ACS Reagent obtained from Sigma-Aldrich), where it remained for 3 h at 70 °C. The surface was
then washed with excessive amount of distilled water for at least 10 min, and was placed in a distilled water bath for about 45 min. Finally, it was dried in the vacuum oven at 80 °C for 15
min. GRAPHENE PREPARATION We fabricated graphene films using a protocol developed by Srivastava _et al_.27 A 25-μm thick Cu foil substrate was loaded into quartz tube and the pressure was
reduced to 10−2 Torr before flowing in Ar/H2 at a pressure of ~8–9 Torr. Heated to 950 °C in Ar/H2 ambient and maintained for 30 min. The Ar/H2 flow was stopped, and hexane vapour evaporated
_in situ_ from a liquid precursor (flow rate ~4 ml h−1) was passed in the quartz tube for 6 min. During the reaction time, we maintained tube pressure of 500 mTorr. Cu foils with the
as-grown graphene films were spin-coated with a thin layer of poly methyl methacrylate (PMMA). Dilute nitric acid was used to dissolve the Cu foil. After dissolution, the PMMA-supported
graphene was carefully washed with deionized (DI) water and transferred onto a silicon wafer capped with 300-nm silicon dioxide layer. The PMMA film was removed using acetone, leaving behind
pure graphene on silicon wafer. To evaluate the number of graphene layers a Renishaw inVia spectrometer was used at 514.5 nm. Raman spectra of graphene are shown in Supplementary Fig. S1.
In addition, TEM image is shown in Supplementary Fig. S2. EXPERIMENTAL PROCEDURE The CAB, used in this study, is described elsewhere4. Water drops were placed on the graphene substrate in a
dust-free laminar flow hood (ULPA filters; Terra Universal) and then the drop-substrate system was carefully placed in the CAB chamber. To suppress the evaporation of the water, we placed
several smaller (‘satellite’) water drops near the main (measured) drop and sealed the chamber as described previously17. Optical images of water drops on the graphene and silane-coated
silicon were taken using Micromaster I microscope from Fisher Scientific. RAMAN SPECTROSCOPY Raman spectroscopy was used to measure graphene thickness and quality. A Renishaw inVia
Microscope was set at 514 nm laser wavelength and at low 0.1 mW power to reduce sample heating. Graphene samples were mounted onto an n-type, 300 nm SiO2 silicon wafer. For each sample, five
different spots were measured and averaged into one curve. The intensity of disorder-induced Raman D-peak at ~1,350 cm−1 is weak, signifying high-quality graphene. The G (~1,595 cm−1) and
2D (~2,695 cm−1) are used to measure graphene layer number29 (_n_) by comparing peak intensities, _I_(_G_)_/I_(_2D_). TEM TEM was done using a JEOL 1,230 High Contrast Schottky-type field
emission gun with a charge-coupled device Gatan model 794 camera. Power was at 120 eV and thermally induced drift was accounted for. Sample preparation was done by dispersing the graphene in
1:1 ethanol water mix and by placing a single drop on a 300-μm holey mesh lacey carbon grid with copper support. After drying, the grid was placed in the TEM chamber and measured. JEOL
company software was used in image analysis. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: N’guessan, H.E. _et al_. Water tribology on graphene. _Nat. Commun._ 3:1242 doi:
10.1038/ncomms2247 (2012). REFERENCES * Tabor D. Friction, surface science and tribology—a personal view. _Proc. Inst. Mech. Eng. C-J. Mech. Eng. Sci._ 205, 365–378 (1991). Article Google
Scholar * Tabor D. Tribology—the last 25 years—a personal view. _Tribol. Int._ 28, 7–10 (1995). Article Google Scholar * Tadmor R. Approaches in wetting phenomena. _Soft Matter_ 7,
1577–1580 (2011). Article CAS ADS Google Scholar * Tadmor R. et al. Measurement of lateral adhesion forces at the interface between a liquid drop and a substrate. _Phys. Rev. Lett._ 103,
266101 (2009). Article ADS Google Scholar * Carré A., Gastel J.-C., Shanahan M. E. R. Viscoelastic effects in the spreading of liquids. _Nature_ 379, 432–434 (1996). Article ADS Google
Scholar * Shanahan M. E. R., Degennes P. G. The ridge produced by a liquid near the triple line solid liquid fluid. _Comptes Rendus Acad. Sci. Serie II_ 302, 517–521 (1986). Google Scholar
* Belman N., Jin K., Golan Y., Israelachvili J. N., Pesika N. S. Origin of the contact angle hysteresis of water on chemisorbed and physisorbed self-assembled monolayers. _Langmuir_ 28,
14609–14617 (2012). Article CAS Google Scholar * Hejazi V., Nyong A. E., Rohatgi P. K., Nosonovsky M. Wetting transitions in underwater oleophobic surface of brass. _Adv. Mater._
doi:10.1002/adma.201202516 (2012). * Bormashenko E., Grynyov R., Bormashenko Y., Drori E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds.
_Scientific Rep._ 2, 741 (2012). Article ADS Google Scholar * Marinescu M., Urbakh M., Barnea T., Kucernak A. R., Kornyshev A. A. Electrowetting dynamics facilitated by pulsing. _J. Phys.
Chem. C_ 114, 22558–22565 (2010). Article CAS Google Scholar * Sahu R. P., Sinha-Ray S., Yarin A. L., Pourdeyhimi B. Drop impacts on electrospun nanofiber membranes. _Soft Matter_ 8,
3957–3970 (2012). Article CAS ADS Google Scholar * Föhlisch A. et al. Direct observation of electron dynamics in the attosecond domain. _Nature_ 436, 373–376 (2005). Article ADS Google
Scholar * Garg A., Han J., Sinnott S. B. Interactions of carbon-nanotubule proximal probe tips with diamond and graphene. _Phys. Rev. Lett._ 81, 2260–2263 (1998). Article CAS ADS Google
Scholar * Lee C. et al. Frictional characteristics of atomically thin sheets. _Science_ 328, 76–80 (2010). Article CAS ADS Google Scholar * Lee C., Wei X. D., Kysar J. W., Hone J.
Measurement of the elastic properties and intrinsic strength of monolayer graphene. _Science_ 321, 385–388 (2008). Article CAS ADS Google Scholar * Lin Y. M. et al. Wafer-scale graphene
integrated circuit. _Science_ 332, 1294–1297 (2011). Article CAS ADS Google Scholar * Tadmor R. et al. Drop retention force as a function of resting time. _Langmuir_ 24, 9370–9374
(2008). Article CAS Google Scholar * Spirin L., Galuschko A., Kreer T., Binder K., Baschnagel J. Polymer-brush lubricated surfaces with colloidal inclusions under shear inversion. _Phys.
Rev. Lett._ 106, 168301 (2011). Article CAS ADS Google Scholar * Tadmor R., Janik J., Klein J., Fetters L. J. Sliding friction with polymer brushes. _Phys. Rev. Lett._ 91, 115503 (2003).
Article ADS Google Scholar * Yu W., Madhukar A. Molecular dynamics study of coherent island energetics, stresses, and strains in highly strained epitaxy. _Phys. Rev. Lett._ 79, 905–908
(1997). Article CAS ADS Google Scholar * Leh A. et al. On the role of the three-phase contact line in surface deformation. _Langmuir_ 28, 5795–5801 (2012). Article CAS Google Scholar
* Yadav P. S., Bahadur P., Tadmor R., Chaurasia K., Leh A. Drop retention force as a function of drop size. _Langmuir_ 24, 3181–3184 (2008). Article CAS Google Scholar * Tadmor R. Line
energy, line tension and drop size. _Surf. Sci._ 602, L108–L111 (2008). Article CAS ADS Google Scholar * de Gennes P.-G., Brochard-Wyart F., Quéré D. _Capillarity and Wetting Phenomena_
Springer-Verlag: New York, (2004). * Fan L. et al. Topology evolution of graphene in chemical vapor deposition, a combined theoretical/experimental approach toward shape control of graphene
domains. _Nanotechnology_ 23, 115605 (2012). Article ADS Google Scholar * Robertson A. W. et al. Atomic structure of interconnected few-layer graphene domains. _ACS Nano_ 5, 6610–6618
(2011). Article CAS Google Scholar * Srivastava A. et al. Novel liquid precursor-based facile synthesis of large-area continuous, single, and few-layer graphene films. _Chem. Mater._ 22,
3457–3461 (2010). Article CAS Google Scholar * Bewig K. W., Zisman W. A. The wetting of gold and platinum by water. _J. Phys. Chem._ 69, 4238–4242 (1965). Article CAS Google Scholar *
Ferrari A. C. et al. Raman spectrum of graphene and graphene layers. _Phys. Rev. Lett._ 97, 187401 (2006). Article CAS ADS Google Scholar Download references ACKNOWLEDGEMENTS R.T.,
H.E.N., A.L., P.B. and P.W. were supported by the National Science Foundation Grants DMR-0619458 and CBET-0960229. R.T., H.E.N. and A.L. were supported by the STLE Houston Section. P.C. was
funded by the NASA through the GSRP programme, grant number NNX10AL39H. P.M.A. was funded by the Office of Naval Research through the MURI programme on Functionalized Nanoscale Graphene,
grant number WFUHS10473. AUTHOR INFORMATION Author notes * Hartmann E. N’guessan, Aisha Leh and Paris Cox: These authors contributed equally to this work AUTHORS AND AFFILIATIONS * Dan F.
Smith Department of Chemical Engineering, Lamar University, P. O. Box 10053, Beaumont, 77710, Texas, USA Hartmann E. N’guessan, Aisha Leh, Prashant Bahadur, Rafael Tadmor & Priyanka
Wasnik * Department of Mechanical Engineering and Materials Science, Rice University, 6100 Main Street, Houston, 77005, Texas, USA Paris Cox, Robert Vajtai & Pulickel M. Ajayan *
Department of Biomedical Engineering, University of Bridgeport, 221 University Avenue, Bridgeport, 06604, Connecticut, USA Prabir Patra * Department of Mechanical Engineering, University of
Bridgeport, 221 University Avenue, Bridgeport, 06604, Connecticut, USA Prabir Patra Authors * Hartmann E. N’guessan View author publications You can also search for this author inPubMed
Google Scholar * Aisha Leh View author publications You can also search for this author inPubMed Google Scholar * Paris Cox View author publications You can also search for this author
inPubMed Google Scholar * Prashant Bahadur View author publications You can also search for this author inPubMed Google Scholar * Rafael Tadmor View author publications You can also search
for this author inPubMed Google Scholar * Prabir Patra View author publications You can also search for this author inPubMed Google Scholar * Robert Vajtai View author publications You can
also search for this author inPubMed Google Scholar * Pulickel M. Ajayan View author publications You can also search for this author inPubMed Google Scholar * Priyanka Wasnik View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS R.T., P.P. and P.A. conceptualized, planned and coordinated the study; H.E.N., A.L., P.C., P.P., P.B.
and R.T. conducted CAB experiments with graphene; H.E.N. and P.W. conducted CAB experiments with HOPG; H.E.N. analysed the CAB data; P.C. and P.P. conducted TEM and Raman experiments; H.E.N.
and A.L. preformed the microscopy experiments; P.C. and R.V. synthesized the graphene; and R.T., H.E.N., A.L., P.C., P.P. and R.V. co-wrote the paper. All authors discussed the results and
commented on the paper. CORRESPONDING AUTHORS Correspondence to Rafael Tadmor or Prabir Patra. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION 1 Supplementary Figures S1-S3 (PDF 368 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE N’guessan,
H., Leh, A., Cox, P. _et al._ Water tribology on graphene. _Nat Commun_ 3, 1242 (2012). https://doi.org/10.1038/ncomms2247 Download citation * Received: 22 August 2012 * Accepted: 01
November 2012 * Published: 04 December 2012 * DOI: https://doi.org/10.1038/ncomms2247 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
