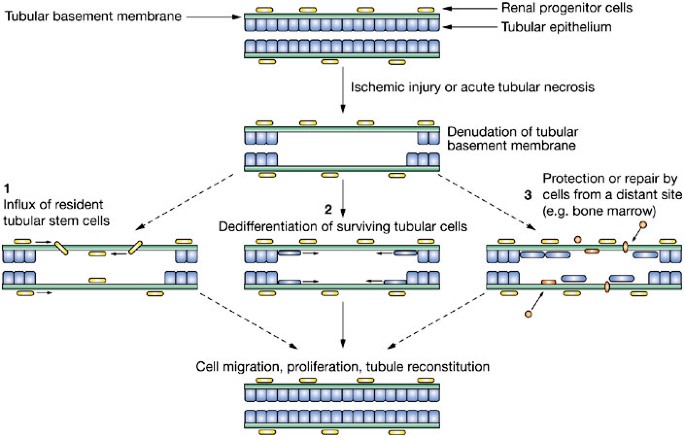
Adult stem cells in the repair of the injured renal tubule
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The capacity of the kidney to regenerate functional tubules following episodes of acute injury is an important determinant of patient morbidity and mortality in the hospital
setting. After severe injury or repeated episodes of injury, kidney recovery can be significantly impaired or even fail completely. Although significant advances have been made in the
clinical management of such cases, there is no specific therapy that can improve the rate or effectiveness of the repair process. Recent studies have indicated that adult stem cells, either
in the kidney itself or derived from the bone marrow, could participate in this repair process and might therefore be utilized clinically to treat acute renal failure. This review will focus
on our current understanding of these stem cells, the controversies surrounding their _in vivo_ capacity to repopulate the renal tubule, and further investigations that will be required
before stem cell therapy can be considered for use in the clinical setting. KEY POINTS * A population of tubule progenitor cells may persist in the renal interstitium of adults * Stem cells
from the bone marrow mobilize to the kidney after injury * Mobilizing or infusing bone-marrow stem cells (BMSCs) can have a protectiveeffect in animal models of acute renal failure * It is
not clear whether BMSCs differentiate to form tubule cells, or fuse with existing tubule elements * The low frequency of BMSC differentiation and fusion make it unlikely that these processes
account for functional recovery of injured tubules Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS
Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS CONCISE REVIEW: CURRENT TRENDS ON APPLICATIONS OF STEM CELLS IN DIABETIC
NEPHROPATHY Article Open access 21 November 2020 REPLACING RENAL FUNCTION USING BIOENGINEERED TISSUES Article 17 May 2023 EFFECTS OF OBESITY ON REPARATIVE FUNCTION OF HUMAN ADIPOSE
TISSUE-DERIVED MESENCHYMAL STEM CELLS ON ISCHEMIC MURINE KIDNEYS Article 07 March 2022 REFERENCES * Bonventre JV (2003) Dedifferentiation and proliferation of surviving epithelial cells in
acute renal failure. _J Am Soc Nephrol_ 14: S55–S61 Article Google Scholar * Schena FP (1998) Role of growth factors in acute renal failure. _Kidney Int_ 66 (Suppl): S11–S15 CAS Google
Scholar * Vainio S and Muller U (1997) Inductive tissue interactions, cell signaling, and the control of kidney organogenesis. _Cell_ 90: 975–978 Article CAS Google Scholar * Salice CJ
et al. (2001) New nephron development in goldfish (Carassius auratus) kidneys following repeated gentamicin-induced nephrotoxicosis. _Comp Med_ 51: 56–59 CAS PubMed Google Scholar * Elger
M et al. (2003) Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. _J Am Soc Nephrol_ 14: 1506–1518 Article Google Scholar * Maeshima A et al. (2003)
Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. _J Am Soc Nephrol_ 14: 3138–3146 Article Google Scholar * Oliver JA et
al. (2004) The renal papilla is a niche for adult kidney stem cells. _J Clin Invest_ 114: 795–804 Article CAS Google Scholar * Cotsarelis G et al. (1989) Existence of slow-cycling limbal
epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. _Cell_ 57: 201–209 Article CAS Google Scholar * Goodell MA et al.
(1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating _in vivo_. _J Exp Med_ 183: 1797–1806 Article CAS Google Scholar * Zhou S et al. (2001)
The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. _Nat Med_ 7: 1028–1034 Article CAS Google
Scholar * Asakura A et al. (2002) Myogenic specification of side population cells in skeletal muscle. _J Cell Biol_ 159: 123–134 Article CAS Google Scholar * Hishikawa K et al. (2005)
Musculin/MyoR is expressed in kidney side population cells and can regulate their function. _J Cell Biol_ 169: 921–928 Article CAS Google Scholar * Uchida N et al. (2000) Direct isolation
of human central nervous system stem cells. _Proc Natl Acad Sci USA_ 97: 14720–14725 Article CAS Google Scholar * Peichev M et al. (2000) Expression of VEGFR-2 and AC133 by circulating
human CD34(+) cells identifies a population of functional endothelial precursors. _Blood_ 95: 952–958 CAS Google Scholar * Corbeil D et al. (2000) The human AC133 hematopoietic stem cell
antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. _J Biol Chem_ 275: 5512–5520 Article CAS Google Scholar * Bussolati B et al. (2005) Isolation of
renal progenitor cells from adult human kidney. _Am J Pathol_ 166: 545–555 Article CAS Google Scholar * Short B et al. (2003) Mesenchymal stem cells. _Arch Med Res_ 34: 565–571 Article
CAS Google Scholar * Challen GA et al. (2004) Identifying the molecular phenotype of renal progenitor cells. _J Am Soc Nephrol_ 15: 2344–2357 Article CAS Google Scholar * Bensidhoum M
et al. (2004) Homing of in vitro expanded Stro-1− or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. _Blood_ 103:
3313–3319 Article CAS Google Scholar * Javazon EH et al. (2004) Mesenchymal stem cells: paradoxes of passaging. _Exp Hematol_ 32: 414–425 Article CAS Google Scholar * Gronthos S et al.
(2003) Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. _J Cell Sci_ 116: 1827–1835 Article CAS Google Scholar * Krause DS et
al. (2001) Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. _Cell_ 105: 369–377 Article CAS Google Scholar * Lagasse E et al. (2000) Purified
hematopoietic stem cells can differentiate into hepatocytes _in vivo_. _Nat Med_ 6: 1229–1234 Article CAS Google Scholar * Ianus A et al. (2003) In vivo derivation of glucose-competent
pancreatic endocrine cells from bone marrow without evidence of cell fusion. _J Clin Invest_ 111: 843–850 Article CAS Google Scholar * Reyes M et al. (2002) Origin of endothelial
progenitors in human postnatal bone marrow. _J Clin Invest_ 109: 337–346 Article CAS Google Scholar * Herzog EL et al. (2003) Plasticity of marrow-derived stem cells. _Blood_ 102:
3483–3493 Article CAS Google Scholar * Wagers AJ et al. (2002) Little evidence for developmental plasticity of adult hematopoietic stem cells. _Science_ 297: 2256–2259 Article CAS
Google Scholar * Wagers AJ and Weissman IL (2004) Plasticity of adult stem cells. _Cell_ 116: 639–648 Article CAS Google Scholar * Kale S et al. (2003) Bone marrow stem cells contribute
to repair of the ischemically injured renal tubule. _J Clin Invest_ 112: 42–49 Article CAS Google Scholar * Togel F et al. (2004) Hematopoietic stem cell mobilization-associated
granulocytosis severely worsens acute renal failure. _J Am Soc Nephrol_ 15: 1261–1267 Article Google Scholar * Togel F et al. (2005) Renal SDF-1 signals mobilization and homing of
CXCR4-positive cells to the kidney after ischemic injury. _Kidney Int_ 67: 1772–1784 Article Google Scholar * Duffield JS et al. (2005) Restoration of tubular epithelial cells during
repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. _J Clin Invest_ 115: 1743–1755 Article CAS Google Scholar * Zhang Y et al. (2004)
Ischemia-reperfusion induces G-CSF gene expression by renal medullary thick ascending limb cells _in vivo_ and _in vitro_. _Am J Physiol Renal Physiol_ 286: F1193–F1201 Article CAS Google
Scholar * Safirstein R et al. (1991) Expression of cytokine-like genes JE and KC is increased during renal ischemia. _Am J Physiol_ 261: F1095–F1101 CAS PubMed Google Scholar * Guidot DM
et al. (1994) Interleukin-1 treatment increases neutrophils but not antioxidant enzyme activity or resistance to ischemia-reperfusion injury in rat kidneys. _Inflammation_ 18: 537–545
Article CAS Google Scholar * Haq M et al. (1998) Role of IL-1 in renal ischemic reperfusion injury. _J Am Soc Nephrol_ 9: 614–619 CAS PubMed Google Scholar * Patel NS et al. (2005)
Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. _J Pharmacol Exp Ther_ 312: 1170–1178 Article CAS Google Scholar *
Ysebaert DK et al. (2004) T cells as mediators in renal ischemia/reperfusion injury. _Kidney Int_ 66: 491–496 Article CAS Google Scholar * Bonventre JV and Zuk A (2004) Ischemic acute
renal failure: an inflammatory disease? _Kidney Int_ 66: 480–485 Article CAS Google Scholar * Poulsom R et al. (2001) Bone marrow contributes to renal parenchymal turnover and
regeneration. _J Pathol_ 195: 229–235 Article CAS Google Scholar * Gupta S et al. (2002) A role for extrarenal cells in the regeneration following acute renal failure. _Kidney Int_ 62:
1285–1290 Article Google Scholar * Fang TC et al. (2005) Proliferation of bone marrow-derived cells contributes to regeneration after folic acid-induced acute tubular injury. _J Am Soc
Nephrol_ 16: 1723–1732 Article CAS Google Scholar * Lin F et al. (2005) Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. _J
Clin Invest_ 115: 1756–1764 Article CAS Google Scholar * Anjos-Afonso F et al. (2004) _In vivo_ contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage
conditions. _J Cell Sci_ 117: 5655–5664 Article CAS Google Scholar * Yokoo T et al. (2005) Human mesenchymal stem cells in rodent whole-embryo culture are reprogrammed to contribute to
kidney tissues. _Proc Natl Acad Sci USA_ 102: 3296–3300 Article CAS Google Scholar * Lin F et al. (2003) Hematopoietic stem cells contribute to the regeneration of renal tubules after
renal ischemia-reperfusion injury in mice. _J Am Soc Nephrol_ 14: 1188–1199 Article Google Scholar * Truong LD et al. (1992) Experimental chronic renal ischemia: morphologic and
immunologic studies. _Kidney Int_ 41: 1676–1689 Article CAS Google Scholar * Krause D and Cantley LG (2005) Bone marrow plasticity revisited: protection or differentiation in the kidney
tubule? _J Clin Invest_ 115: 1705–1708 Article CAS Google Scholar * Szczypka MS et al. (2005) Rare incorporation of bone marrow-derived cells into kidney after folic acid-induced injury.
_Stem Cells_ 23: 44–54 Article CAS Google Scholar * Morigi M et al. (2004) Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure.
_J Am Soc Nephrol_ 15: 1794–1804 Article Google Scholar * Herrera MB et al. (2004) Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. _Int J Mol
Med_ 14: 1035–1041 PubMed Google Scholar * Togel F et al. (2005) Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent
mechanisms. _Am J Physiol Renal Physiol_ 289: F31–42 Article Google Scholar * Stokman G et al. (2005) Hematopoietic stem cell mobilization therapy accelerates recovery of renal function
independent of stem cell contribution. _J Am Soc Nephrol_ 16: 1684–1692 Article CAS Google Scholar * Iwasaki M et al. (2005) Mobilization of bone marrow cells by G-CSF rescues mice from
cisplatin-induced renal failure, and M-CSF enhances the effects of G-CSF. _J Am Soc Nephrol_ 16: 658–666 Article CAS Google Scholar * Harada M et al. (2005) G-CSF prevents cardiac
remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. _Nat Med_ 11: 305–311 Article CAS Google Scholar * Aggarwal S and Pittenger MF (2005) Human
mesenchymal stem cells modulate allogeneic immune cell responses. _Blood_ 105: 1815–1822 Article CAS Google Scholar * Sharples EJ et al. (2004) Erythropoietin protects the kidney against
the injury and dysfunction caused by ischemia-reperfusion. _J Am Soc Nephrol_ 15: 2115–2124 Article CAS Google Scholar * Patel NS et al. (2004) Pretreatment with EPO reduces the injury
and dysfunction caused by ischemia/reperfusion in the mouse kidney _in vivo_. _Kidney Int_ 66: 983–989 Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The author would
like to thank M Egalka for the images in Figure 3, and D Krause for her helpful comments on the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * an Associate Professor in the
Section of Nephrology at Yale University School of Medicine, New Haven, CT, USA Lloyd G Cantley Authors * Lloyd G Cantley View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Lloyd G Cantley. ETHICS DECLARATIONS COMPETING INTERESTS The author declares no competing financial interests. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Cantley, L. Adult stem cells in the repair of the injured renal tubule. _Nat Rev Nephrol_ 1, 22–32 (2005).
https://doi.org/10.1038/ncpneph0021 Download citation * Received: 17 May 2005 * Accepted: 01 September 2005 * Issue Date: 01 November 2005 * DOI: https://doi.org/10.1038/ncpneph0021 SHARE
THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative
