The receptor S1P1 overrides regulatory T cell–mediated immune suppression through Akt-mTOR
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

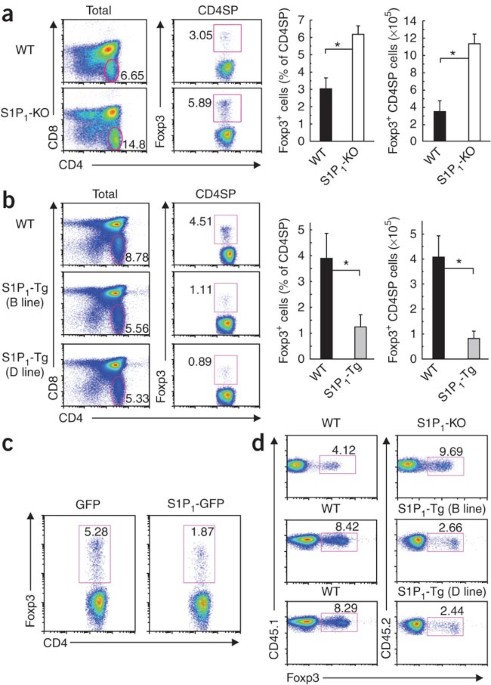
Regulatory T cells (Treg cells) are critically involved in maintaining immunological tolerance, but this potent suppression must be 'quenched' to allow the generation of adaptive immune
responses. Here we report that sphingosine 1-phosphate (S1P) receptor type 1 (S1P1) delivers an intrinsic negative signal to restrain the thymic generation, peripheral maintenance and
suppressive activity of Treg cells. Combining loss- and gain-of-function genetic approaches, we found that S1P1 blocked the differentiation of thymic Treg precursors and function of mature
Treg cells and affected Treg cell–mediated immune tolerance. S1P1 induced selective activation of the Akt-mTOR kinase pathway to impede the development and function of Treg cells. Dynamic
regulation of S1P1 contributed to lymphocyte priming and immune homeostasis. Thus, by antagonizing Treg cell–mediated immune suppression, the lipid-activated S1P1-Akt-mTOR pathway
orchestrates adaptive immune responses.
We thank A. Rudensky (University of Washington) for Foxp3gfp 'knock-in' mice; D. Hildeman (University of Cincinnati) for dominant negative and constitutively active Akt retroviral
constructs; S. Shrestha for help with genotyping; M. McGargill for help with the FTOC procedure; R. Cross and G. Lennon for cell sorting; and D. Green and D. Vignali for scientific
discussions and reagents. Supported by the US National Institutes of Health (H.C.), the Arthritis Foundation (H.C.), the Arthritis National Research Foundation (H.C.), the American Lebanese
Syrian Associated Charities (H.C.) and the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R.L.P.).
Department of Immunology, St. Jude Children's Research Hospital, Memphis, Tennessee, USA
Animal Resources Center, St. Jude Children's Research Hospital, Memphis, Tennessee, USA
Genetics of Development and Disease Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, USA
Howard Hughes Medical Institute, New Haven, Connecticut, USA
Department of Immunobiology, Yale University School of Medicine, New Haven, Connecticut, USA
G.L. designed and did the experiments with cells and mice, analyzed data and contributed to writing the manuscript; S.B. did retroviral transduction of bone marrow cells and reconstitution
and managed the mouse colony; G.H. and S.B. contributed to real-time PCR analysis; K.B. analyzed and assigned scores to histology data; R.L.P. and R.A.F. provided animal models; and H.C.
designed experiments, analyzed data, wrote the manuscript and provided overall direction.
Anyone you share the following link with will be able to read this content:
