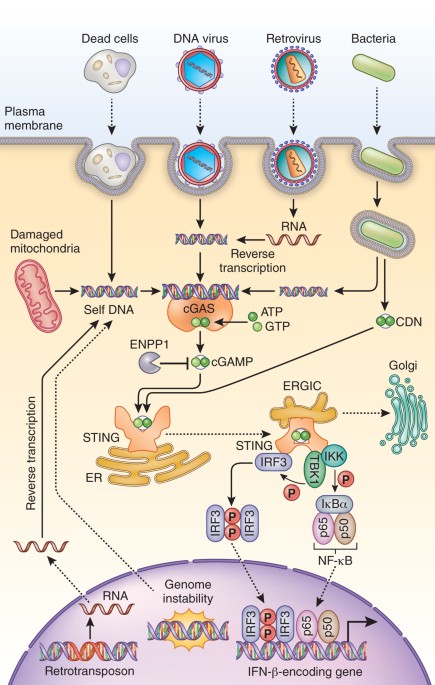
Regulation and function of the cgas–sting pathway of cytosolic dna sensing
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The recognition of microbial nucleic acids is a major mechanism by which the immune system detects pathogens. Cyclic GMP-AMP (cGAMP) synthase (cGAS) is a cytosolic DNA sensor that
activates innate immune responses through production of the second messenger cGAMP, which activates the adaptor STING. The cGAS–STING pathway not only mediates protective immune defense
against infection by a large variety of DNA-containing pathogens but also detects tumor-derived DNA and generates intrinsic antitumor immunity. However, aberrant activation of the cGAS
pathway by self DNA can also lead to autoimmune and inflammatory disease. Thus, the cGAS pathway must be properly regulated. Here we review the recent advances in understanding of the
cGAS–STING pathway, focusing on the regulatory mechanisms and roles of this pathway in heath and disease. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS CROSSTALK BETWEEN CGAS–STING SIGNALING
AND CELL DEATH Article 18 September 2020 CYTOSOLIC DNA SENSING BY CGAS: REGULATION, FUNCTION, AND HUMAN DISEASES Article Open access 30 April 2021 CGAMP-ACTIVATED CGAS–STING SIGNALING: ITS
BACTERIAL ORIGINS AND EVOLUTIONARY ADAPTATION BY METAZOANS Article 09 March 2023 REFERENCES * Pandey, S., Kawai, T. & Akira, S. Microbial sensing by Toll-like receptors and intracellular
nucleic acid sensors. _Cold Spring Harb. Perspect. Biol._ 7, a016246 (2014). PubMed Google Scholar * Broz, P. & Dixit, V.M. Inflammasomes: mechanism of assembly, regulation and
signalling. _Nat. Rev. Immunol._ 16, 407–420 (2016). CAS PubMed Google Scholar * Yoneyama, M., Onomoto, K., Jogi, M., Akaboshi, T. & Fujita, T. Viral RNA detection by RIG-I-like
receptors. _Curr. Opin. Immunol._ 32, 48–53 (2015). CAS PubMed Google Scholar * Cai, X., Chiu, Y.H. & Chen, Z.J. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling.
_Mol. Cell_ 54, 289–296 (2014). CAS PubMed Google Scholar * Land, W.G. _Innate Alloimmunity, Part 1: Innate Immunity and Host Defense_ (Pabst Science Publishers, 2011). Google Scholar *
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. _Science_ 339, 786–791 (2013). CAS PubMed
Google Scholar * Zhang, X. et al. The cytosolic DNA sensor cGAS forms an oligomeric complex with DNA and undergoes switch-like conformational changes in the activation loop. _Cell Rep._
6, 421–430 (2014). CAS PubMed PubMed Central Google Scholar * Li, X. et al. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. _Immunity_ 39, 1019–1031
(2013). CAS PubMed Google Scholar * Kranzusch, P.J., Lee, A.S., Berger, J.M. & Doudna, J.A. Structure of human cGAS reveals a conserved family of second-messenger enzymes in innate
immunity. _Cell Reports_ 3, 1362–1368 (2013). CAS PubMed Google Scholar * Gao, P. et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic
GMP-AMP synthase. _Cell_ 153, 1094–1107 (2013). CAS PubMed PubMed Central Google Scholar * Civril, F. et al. Structural mechanism of cytosolic DNA sensing by cGAS. _Nature_ 498, 332–337
(2013). CAS PubMed PubMed Central Google Scholar * Wu, J. et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. _Science_ 339, 826–830
(2013). CAS PubMed Google Scholar * Zhang, X. et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. _Mol. Cell_ 51, 226–235
(2013). CAS PubMed Google Scholar * Diner, E.J. et al. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. _Cell Reports_ 3,
1355–1361 (2013). CAS PubMed Google Scholar * Ablasser, A. et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. _Nature_ 498, 380–384 (2013). CAS
PubMed PubMed Central Google Scholar * Ishikawa, H. & Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. _Nature_ 455, 674–678 (2008).
Article CAS PubMed PubMed Central Google Scholar * Zhong, B. et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. _Immunity_ 29,
538–550 (2008). CAS PubMed Google Scholar * Saitoh, T. et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. _Proc. Natl. Acad. Sci. USA_ 106,
20842–20846 (2009). CAS PubMed PubMed Central Google Scholar * Ishikawa, H., Ma, Z. & Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate
immunity. _Nature_ 461, 788–792 (2009). CAS PubMed PubMed Central Google Scholar * Dobbs, N. et al. STING activation by translocation from the ER is associated with infection and
autoinflammatory disease. _Cell Host Microbe_ 18, 157–168 (2015). CAS PubMed PubMed Central Google Scholar * Tanaka, Y. & Chen, Z.J. STING specifies IRF3 phosphorylation by TBK1 in
the cytosolic DNA signaling pathway. _Sci. Signal._ 5, ra20 (2012). PubMed PubMed Central Google Scholar * Fitzgerald, K.A. et al. IKKe and TBK1 are essential components of the IRF3
signaling pathway. _Nat. Immunol._ 4, 491–496 (2003). CAS PubMed Google Scholar * Sharma, S. et al. Triggering the interferon antiviral response through an IKK-related pathway. _Science_
300, 1148–1151 (2003). CAS PubMed Google Scholar * Herzner, A.-M. et al. Sequence-specific activation of the DNA sensor cGAS by Y-form DNA structures as found in primary HIV-1 cDNA. _Nat.
Immunol._ 16, 1025–1033 (2015). CAS PubMed PubMed Central Google Scholar * Gehrke, N. et al. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and
potentiates STING-dependent immune sensing. _Immunity_ 39, 482–495 (2013). CAS PubMed Google Scholar * Seo, G.J. et al. Akt kinase-mediated checkpoint of cGAS DNA sensing pathway. _Cell
Rep._ 13, 440–449 (2015). CAS PubMed PubMed Central Google Scholar * Xia, P. et al. Glutamylation of the DNA sensor cGAS regulates its binding and synthase activity in antiviral
immunity. _Nat. Immunol._ 17, 369–378 (2016). CAS PubMed Google Scholar * Schoggins, J.W. et al. A diverse range of gene products are effectors of the type I interferon antiviral
response. _Nature_ 472, 481–485 (2011). CAS PubMed PubMed Central Google Scholar * Ma, F. et al. Positive feedback regulation of type I IFN production by the IFN-inducible DNA sensor
cGAS. _J. Immunol._ 194, 1545–1554 (2015). CAS PubMed Google Scholar * Chiu, Y.H., Macmillan, J.B. & Chen, Z.J. RNA polymerase III detects cytosolic DNA and induces type I interferons
through the RIG-I pathway. _Cell_ 138, 576–591 (2009). CAS PubMed PubMed Central Google Scholar * Xia, T., Konno, H., Ahn, J. & Barber, G.N. Deregulation of STING signaling in
colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. _Cell Rep._ 14, 282–297 (2016). CAS PubMed Google Scholar * Thomsen, M.K. et al. Lack of
immunological DNA sensing in hepatocytes facilitates hepatitis B virus infection. _Hepatology_ 64, 746–759 (2016). CAS PubMed Google Scholar * Berg, R.K. et al. T cells detect
intracellular DNA but fail to induce type I IFN responses: implications for restriction of HIV replication. _PLoS One_ 9, e84513 (2014). PubMed PubMed Central Google Scholar * Li, L. et
al. Hydrolysis of 2′3′-cGAMP by ENPP1 and design of nonhydrolyzable analogs. _Nat. Chem. Biol._ 10, 1043–1048 (2014). CAS PubMed PubMed Central Google Scholar * Ablasser, A. et al. Cell
intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. _Nature_ 503, 530–534 (2013). CAS PubMed PubMed Central Google Scholar * Gentili, M. et al.
Transmission of innate immune signaling by packaging of cGAMP in viral particles. _Science_ 349, 1232–1236 (2015). CAS PubMed Google Scholar * Bridgeman, A. et al. Viruses transfer the
antiviral second messenger cGAMP between cells. _Science_ 349, 1228–1232 (2015). CAS PubMed PubMed Central Google Scholar * Gao, P. et al. Structure-function analysis of STING activation
by c[G(2′,5′)pA(3′,5′)p] and targeting by antiviral DMXAA. _Cell_ 154, 748–762 (2013). CAS PubMed PubMed Central Google Scholar * Yin, Q. et al. Cyclic di-GMP sensing via the innate
immune signaling protein STING. _Mol. Cell_ 46, 735–745 (2012). CAS PubMed PubMed Central Google Scholar * Shu, C., Yi, G., Watts, T., Kao, C.C. & Li, P. Structure of STING bound to
cyclic di-GMP reveals the mechanism of cyclic dinucleotide recognition by the immune system. _Nat. Struct. Mol. Biol._ 19, 722–724 (2012). CAS PubMed PubMed Central Google Scholar *
Shang, G. et al. Crystal structures of STING protein reveal basis for recognition of cyclic di-GMP. _Nat. Struct. Mol. Biol._ 19, 725–727 (2012). CAS PubMed Google Scholar * Ouyang, S. et
al. Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. _Immunity_ 36, 1073–1086 (2012). CAS PubMed Google Scholar *
Tsuchiya, Y., Jounai, N., Takeshita, F., Ishii, K.J. & Mizuguchi, K. Ligand-induced ordering of the C-terminal tail primes STING for phosphorylation by TBK1. _EBioMedicine_ 9, 87–96
(2016). PubMed PubMed Central Google Scholar * Shi, H., Wu, J., Chen, Z.J. & Chen, C. Molecular basis for the specific recognition of the metazoan cyclic GMP-AMP by the innate immune
adaptor protein STING. _Proc. Natl. Acad. Sci. USA_ 112, 8947–8952 (2015). CAS PubMed PubMed Central Google Scholar * Kim, S. et al. Anticancer flavonoids are mouse-selective STING
agonists. _ACS Chem. Biol._ 8, 1396–1401 (2013). CAS PubMed PubMed Central Google Scholar * Conlon, J. et al. Mouse, but not human STING, binds and signals in response to the vascular
disrupting agent 5,6-dimethylxanthenone-4-acetic acid. _J. Immunol._ 190, 5216–5225 (2013). CAS PubMed Google Scholar * Cavlar, T., Deimling, T., Ablasser, A., Hopfner, K.P. &
Hornung, V. Species-specific detection of the antiviral small-molecule compound CMA by STING. _EMBO J._ 32, 1440–1450 (2013). CAS PubMed PubMed Central Google Scholar * Liu, S. et al.
Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. _Science_ 347, aaa2630 (2015). PubMed Google Scholar * Konno, H., Konno, K. & Barber,
G.N. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. _Cell_ 155, 688–698 (2013). CAS PubMed Google Scholar * Zhang, J., Hu,
M.M., Wang, Y.Y. & Shu, H.B. TRIM32 protein modulates type I interferon induction and cellular antiviral response by targeting MITA/STING protein for K63-linked ubiquitination. _J.
Biol. Chem._ 287, 28646–28655 (2012). CAS PubMed PubMed Central Google Scholar * Tsuchida, T. et al. The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular
double-stranded DNA. _Immunity_ 33, 765–776 (2010). CAS PubMed Google Scholar * Wang, Q. et al. The E3 ubiquitin ligase AMFR and INSIG1 bridge the activation of TBK1 kinase by modifying
the adaptor STING. _Immunity_ 41, 919–933 (2014). CAS PubMed Google Scholar * Zhong, B. et al. The ubiquitin ligase RNF5 regulates antiviral responses by mediating degradation of the
adaptor protein MITA. _Immunity_ 30, 397–407 (2009). CAS PubMed Google Scholar * Wang, Y. et al. TRIM30a Is a negative-feedback regulator of the intracellular DNA and DNA virus-triggered
response by targeting STING. _PLoS Pathog._ 11, e1005012 (2015). PubMed PubMed Central Google Scholar * Mukai, K. et al. Activation of STING requires palmitoylation at the Golgi. _Nat.
Commun._ 7, 11932 (2016). CAS PubMed PubMed Central Google Scholar * Paludan, S.R. & Bowie, A.G. Immune sensing of DNA. _Immunity_ 38, 870–880 (2013). CAS PubMed PubMed Central
Google Scholar * Ishii, K.J. et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. _Nature_ 451, 725–729 (2008). CAS PubMed Google Scholar * Gray,
E.E. et al. The AIM2-like receptors are dispensable for the interferon response to intracellular DNA. _Immunity_ 45, 255–266 (2016). CAS PubMed PubMed Central Google Scholar * Yoh, S.M.
et al. PQBP1 is a proximal sensor of the cGAS-dependent innate response to HIV-1. _Cell_ 161, 1293–1305 (2015). CAS PubMed PubMed Central Google Scholar * Liang, Q. et al. Crosstalk
between the cGAS DNA sensor and Beclin-1 autophagy protein shapes innate antimicrobial immune responses. _Cell Host Microbe_ 15, 228–238 (2014). CAS PubMed PubMed Central Google Scholar
* Paijo, J. et al. cGAS senses human cytomegalovirus and induces type I interferon responses in human monocyte-derived cells. _PLoS Pathog._ 12, e1005546 (2016). PubMed PubMed Central
Google Scholar * Lio, C.W. et al. cGAS-STING signaling regulates initial innate control of cytomegalovirus infection. _J. Virol._ 90, 7789–7797 (2016). CAS PubMed PubMed Central Google
Scholar * Zhang, G. et al. Cytoplasmic isoforms of Kaposi sarcoma herpesvirus LANA recruit and antagonize the innate immune DNA sensor cGAS. _Proc. Natl. Acad. Sci. USA_ 113, E1034–E1043
(2016). CAS PubMed PubMed Central Google Scholar * Wu, J.J. et al. Inhibition of cGAS DNA sensing by a herpesvirus virion protein. _Cell Host Microbe_ 18, 333–344 (2015). CAS PubMed
PubMed Central Google Scholar * Ma, Z. et al. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. _Proc. Natl. Acad. Sci. USA_ 112, E4306–E4315 (2015). CAS PubMed
PubMed Central Google Scholar * Li, X.D. et al. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. _Science_ 341, 1390–1394 (2013). CAS PubMed Google
Scholar * Schoggins, J.W. et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. _Nature_ 505, 691–695 (2014). CAS PubMed Google Scholar *
Holm, C.K. et al. Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. _Nat. Immunol._ 13, 737–743 (2012). CAS PubMed PubMed Central Google Scholar *
Rasaiyaah, J. et al. HIV-1 evades innate immune recognition through specific cofactor recruitment. _Nature_ 503, 402–405 (2013). CAS PubMed PubMed Central Google Scholar * Lahaye, X. et
al. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. _Immunity_ 39, 1132–1142 (2013). CAS PubMed Google Scholar *
Gao, D. et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. _Science_ 341, 903–906 (2013). CAS PubMed Google Scholar * Zeng, M. et al. MAVS, cGAS, and
endogenous retroviruses in T-independent B cell responses. _Science_ 346, 1486–1492 (2014). CAS PubMed PubMed Central Google Scholar * Portnoy, D.A., Auerbuch, V. & Glomski, I.J.
The cell biology of _Listeria monocytogenes_ infection: the intersection of bacterial pathogenesis and cell-mediated immunity. _J. Cell Biol._ 158, 409–414 (2002). CAS PubMed PubMed
Central Google Scholar * Watson, R.O. et al. The cytosolic sensor cGAS detects _Mycobacterium tuberculosis_ DNA to induce type I interferons and activate autophagy. _Cell Host Microbe_ 17,
811–819 (2015). CAS PubMed PubMed Central Google Scholar * Wassermann, R. et al. _Mycobacterium tuberculosis_ differentially activates cGAS- and inflammasome-dependent intracellular
immune responses through ESX-1. _Cell Host Microbe_ 17, 799–810 (2015). CAS PubMed Google Scholar * Collins, A.C. et al. Cyclic GMP-AMP synthase is an innate immune DNA sensor for
_Mycobacterium tuberculosis_. _Cell Host Microbe_ 17, 820–828 (2015). CAS PubMed PubMed Central Google Scholar * Hansen, K. et al. _Listeria monocytogenes_ induces IFNb expression
through an IFI16-, cGAS- and STING-dependent pathway. _EMBO J._ 33, 1654–1666 (2014). CAS PubMed PubMed Central Google Scholar * Storek, K.M., Gertsvolf, N.A., Ohlson, M.B. & Monack,
D.M. cGAS and Ifi204 cooperate to produce type I IFNs in response to _Francisella_ infection. _J. Immunol._ 194, 3236–3245 (2015). CAS PubMed PubMed Central Google Scholar * Zhang, Y.
et al. The DNA sensor, cyclic GMP-AMP synthase, is essential for induction of IFN-b during _Chlamydia trachomatis_ infection. _J. Immunol._ 193, 2394–2404 (2014). CAS PubMed Google Scholar
* Andrade, W.A. et al. Type I interferon induction by _Neisseria gonorrhoeae_: dual requirement of cyclic GMP-AMP synthase and Toll-like receptor 4. _Cell Rep._ 15, 2438–2448 (2016). CAS
PubMed PubMed Central Google Scholar * Andrade, W.A. et al. Group B streptococcus degrades cyclic-di-AMP to modulate STING-dependent type I interferon production. _Cell Host Microbe_ 20,
49–59 (2016). CAS PubMed PubMed Central Google Scholar * Christensen, M.H. et al. HSV-1 ICP27 targets the TBK1-activated STING signalsome to inhibit virus-induced type I IFN expression.
_EMBO J._ 35, 1385–1399 (2016). CAS PubMed PubMed Central Google Scholar * Lau, L., Gray, E.E., Brunette, R.L. & Stetson, D.B. DNA tumor virus oncogenes antagonize the cGAS-STING
DNA-sensing pathway. _Science_ 350, 568–571 (2015). CAS PubMed Google Scholar * Crow, Y.J. Type I interferonopathies: mendelian type I interferon up-regulation. _Curr. Opin. Immunol._ 32,
7–12 (2015). CAS PubMed Google Scholar * Gray, E.E., Treuting, P.M., Woodward, J.J. & Stetson, D.B. Cutting edge: cGAS is required for lethal autoimmune disease in the
Trex1-deficient mouse model of Aicardi-Goutières syndrome. _J. Immunol._ 195, 1939–1943 (2015). CAS PubMed Google Scholar * Gao, D. et al. Activation of cyclic GMP-AMP synthase by
self-DNA causes autoimmune diseases. _Proc. Natl. Acad. Sci. USA_ 112, E5699–E5705 (2015). CAS PubMed PubMed Central Google Scholar * Gall, A. et al. Autoimmunity initiates in
nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. _Immunity_ 36, 120–131 (2012). CAS PubMed PubMed Central Google Scholar * Pokatayev,
V. et al. RNase H2 catalytic core Aicardi-Goutières syndrome-related mutant invokes cGAS-STING innate immune-sensing pathway in mice. _J. Exp. Med._ 213, 329–336 (2016). CAS PubMed PubMed
Central Google Scholar * Mackenzie, K.J. et al. Ribonuclease H2 mutations induce a cGAS/STING-dependent innate immune response. _EMBO J._ 35, 831–844 (2016). CAS PubMed PubMed Central
Google Scholar * Lindahl, T., Barnes, D.E., Yang, Y.G. & Robins, P. Biochemical properties of mammalian TREX1 and its association with DNA replication and inherited inflammatory
disease. _Biochem. Soc. Trans._ 37, 535–538 (2009). CAS PubMed Google Scholar * Yang, Y.G., Lindahl, T. & Barnes, D.E. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint
activation and autoimmune disease. _Cell_ 131, 873–886 (2007). CAS PubMed Google Scholar * Kawane, K. et al. Requirement of DNase II for definitive erythropoiesis in the mouse fetal
liver. _Science_ 292, 1546–1549 (2001). CAS PubMed Google Scholar * Yoshida, H., Okabe, Y., Kawane, K., Fukuyama, H. & Nagata, S. Lethal anemia caused by interferon-beta produced in
mouse embryos carrying undigested DNA. _Nat. Immunol._ 6, 49–56 (2005). CAS PubMed Google Scholar * Okabe, Y., Kawane, K., Akira, S., Taniguchi, T. & Nagata, S. Toll-like
receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. _J. Exp. Med._ 202, 1333–1339 (2005). CAS PubMed PubMed Central Google
Scholar * Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. _N. Engl. J. Med._ 371, 507–518 (2014). CAS PubMed PubMed Central Google Scholar * Dunn, G.P., Koebel,
C.M. & Schreiber, R.D. Interferons, immunity and cancer immunoediting. _Nat. Rev. Immunol._ 6, 836–848 (2006). CAS PubMed Google Scholar * Fuertes, M.B., Woo, S.R., Burnett, B., Fu,
Y.X. & Gajewski, T.F. Type I interferon response and innate immune sensing of cancer. _Trends Immunol._ 34, 67–73 (2013). CAS PubMed Google Scholar * Corrales, L. & Gajewski, T.F.
Endogenous and pharmacologic targeting of the STING pathway in cancer immunotherapy. _Cytokine_ 77, 245–247 (2016). PubMed Google Scholar * Woo, S.R. et al. STING-dependent cytosolic DNA
sensing mediates innate immune recognition of immunogenic tumors. _Immunity_ 41, 830–842 (2014). CAS PubMed PubMed Central Google Scholar * Deng, L. et al. STING-dependent cytosolic DNA
sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. _Immunity_ 41, 843–852 (2014). CAS PubMed PubMed Central Google Scholar * Liu, X.
et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. _Nat. Med._ 21, 1209–1215 (2015). CAS PubMed PubMed Central Google Scholar * Demaria, O. et al. STING
activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. _Proc. Natl. Acad. Sci. USA_ 112, 15408–15413 (2015). CAS PubMed PubMed Central Google
Scholar * Corrales, L. et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. _Cell Rep._ 11, 1018–1030 (2015). CAS
PubMed PubMed Central Google Scholar * Huang, L. et al. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. _J. Immunol._ 191,
3509–3513 (2013). CAS PubMed Google Scholar * Lemos, H. et al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. _Cancer Res._ 76, 2076–2081
(2016). CAS PubMed PubMed Central Google Scholar * Chen, Q. et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. _Nature_ 533, 493–498 (2016). CAS PubMed
PubMed Central Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * The Key Laboratory of Innate Immune Biology of Fujian Province, Biomedical Research Center
of South China, College of Life Sciences, Fujian Normal University, Fuzhou, China Qi Chen * Department of Molecular Biology, University of Texas Southwestern Medical Center, Dallas, Texas,
USA Lijun Sun & Zhijian J Chen * Howard Hughes Medical Institute, University of Texas Southwestern Medical Center, Dallas, Texas, USA Lijun Sun & Zhijian J Chen Authors * Qi Chen
View author publications You can also search for this author inPubMed Google Scholar * Lijun Sun View author publications You can also search for this author inPubMed Google Scholar *
Zhijian J Chen View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Zhijian J Chen. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing financial interests. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chen, Q., Sun, L. & Chen, Z.
Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. _Nat Immunol_ 17, 1142–1149 (2016). https://doi.org/10.1038/ni.3558 Download citation * Received: 18 July 2016 *
Accepted: 16 August 2016 * Published: 20 September 2016 * Issue Date: October 2016 * DOI: https://doi.org/10.1038/ni.3558 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
