Monoclonal antibody targeting of n-cadherin inhibits prostate cancer growth, metastasis and castration resistance
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The transition from androgen-dependent to castration-resistant prostate cancer (CRPC) is a lethal event of uncertain molecular etiology. Comparing gene expression in isogenic
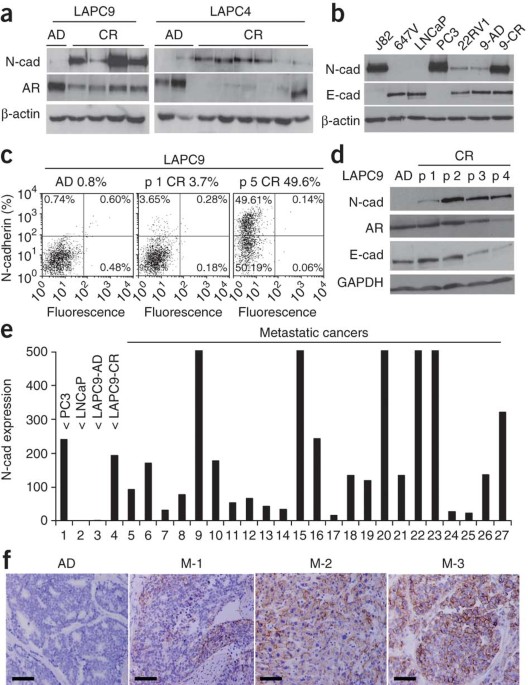
androgen-dependent and CRPC xenografts, we found a reproducible increase in N-cadherin expression, which was also elevated in primary and metastatic tumors of individuals with CRPC. Ectopic
expression of N-cadherin in nonmetastatic, androgen-dependent prostate cancer models caused castration resistance, invasion and metastasis. Monoclonal antibodies against the ectodomain of
N-cadherin reduced proliferation, adhesion and invasion of prostate cancer cells _in vitro_. _In vivo_, these antibodies slowed the growth of multiple established CRPC xenografts, blocked
local invasion and metastasis and, at higher doses, led to complete regression. N-cadherin–specific antibodies markedly delayed the time to emergence of castration resistance, markedly
affected tumor histology and angiogenesis, and reduced both AKT serine-threonine kinase activity and serum interleukin-8 (IL-8) secretion. These data indicate that N-cadherin is a major
cause of both prostate cancer metastasis and castration resistance. Therapeutic targeting of this factor with monoclonal antibodies may have considerable clinical benefit. Access through
your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12
print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be
subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR
CONTENT BEING VIEWED BY OTHERS ANDROGEN RECEPTOR-INDUCED INTEGRIN Α6Β1 AND BNIP3 PROMOTE SURVIVAL AND RESISTANCE TO PI3K INHIBITORS IN CASTRATION-RESISTANT PROSTATE CANCER Article 21 June
2020 LOSS AND REVIVAL OF ANDROGEN RECEPTOR SIGNALING IN ADVANCED PROSTATE CANCER Article Open access 08 January 2021 ANDROGEN RECEPTOR MONOMERS AND DIMERS REGULATE OPPOSING BIOLOGICAL
PROCESSES IN PROSTATE CANCER CELLS Article Open access 03 September 2024 REFERENCES * Chen, C.D. et al. Molecular determinants of resistance to antiandrogen therapy. _Nat. Med._ 10, 33–39
(2004). Article Google Scholar * Suzuki, H., Ueda, T., Ichikawa, T. & Ito, H. Androgen receptor involvement in the progression of prostate cancer. _Endocr. Relat. Cancer_ 10, 209–216
(2003). Article CAS Google Scholar * Mellado, B., Codony, J., Ribal, M.J., Visa, L. & Gascon, P. Molecular biology of androgen-independent prostate cancer: the role of the androgen
receptor pathway. _Clin. Transl. Oncol._ 11, 5–10 (2009). Article CAS Google Scholar * Harris, W.P., Mostaghel, E.A., Nelson, P.S. & Montgomery, B. Androgen deprivation therapy:
progress in understanding mechanisms of resistance and optimizing androgen depletion. _Nat. Clin. Pract. Urol._ 6, 76–85 (2009). Article CAS Google Scholar * Roudier, M.P. et al.
Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. _Hum. Pathol._ 34, 646–653 (2003). Article Google Scholar * Shah, R.B. et al. Androgen-independent prostate
cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. _Cancer Res._ 64, 9209–9216 (2004). Article CAS Google Scholar * Lassi, K. & Dawson, N.A. Emerging
therapies in castrate-resistant prostate cancer. _Curr. Opin. Oncol._ 21, 260–265 (2009). Article CAS Google Scholar * Attard, G., Reid, A.H., Olmos, D. & de Bono, J.S. Antitumor
activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. _Cancer Res._ 69, 4937–4940 (2009). Article CAS Google Scholar * Rizzi,
F. & Bettuzzi, S. Targeting clusterin in prostate cancer. _J. Physiol. Pharmacol._ 59 Suppl 9, 265–274 (2008). PubMed Google Scholar * Gleave, M., Miyake, H. & Chi, K. Beyond
simple castration: targeting the molecular basis of treatment resistance in advanced prostate cancer. _Cancer Chemother. Pharmacol._ 56 Suppl 1, 47–57 (2005). Article Google Scholar *
Sharifi, N., Kawasaki, B.T., Hurt, E.M. & Farrar, W.L. Stem cells in prostate cancer: resolving the castrate-resistant conundrum and implications for hormonal therapy. _Cancer Biol.
Ther._ 5, 901–906 (2006). Article CAS Google Scholar * Isaacs, J.T. The biology of hormone refractory prostate cancer. Why does it develop? _Urol. Clin. North Am._ 26, 263–273 (1999).
Article CAS Google Scholar * Gu, Z. et al. Reg IV: a promising marker of hormone refractory metastatic prostate cancer. _Clin. Cancer Res._ 11, 2237–2243 (2005). Article CAS Google
Scholar * Tso, C.L. et al. Androgen deprivation induces selective outgrowth of aggressive hormone-refractory prostate cancer clones expressing distinct cellular and molecular properties not
present in parental androgen-dependent cancer cells. _Cancer J._ 6, 220–233 (2000). CAS PubMed Google Scholar * Tran, N.L., Adams, D.G., Vaillancourt, R.R. & Heimark, R.L. Signal
transduction from N-cadherin increases Bcl-2. Regulation of the phosphatidylinositol 3-kinase/Akt pathway by homophilic adhesion and actin cytoskeletal organization. _J. Biol. Chem._ 277,
32905–32914 (2002). Article CAS Google Scholar * Araki, S. et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. _Cancer Res._ 67,
6854–6862 (2007). Article CAS Google Scholar * Domingo-Domenech, J. et al. Interleukin 6, a nuclear factor-κB target, predicts resistance to docetaxel in hormone-independent prostate
cancer and nuclear factor-κB inhibition by PS-1145 enhances docetaxel antitumor activity. _Clin. Cancer Res._ 12, 5578–5586 (2006). Article CAS Google Scholar * Tomita, K. et al. Cadherin
switching in human prostate cancer progression. _Cancer Res._ 60, 3650–3654 (2000). CAS PubMed Google Scholar * Jaggi, M. et al. N-cadherin switching occurs in high Gleason grade
prostate cancer. _Prostate_ 66, 193–199 (2006). Article CAS Google Scholar * Gravdal, K., Halvorsen, O.J., Haukaas, S.A. & Akslen, L.A. A switch from E-cadherin to N-cadherin
expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. _Clin. Cancer Res._ 13, 7003–7011 (2007). Article
CAS Google Scholar * Mani, S.A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. _Cell_ 133, 704–715 (2008). Article CAS Google Scholar *
Mason, M.J., Fan, G., Plath, K., Zhou, Q. & Horvath, S. Signed weighted gene co-expression network analysis of transcriptional regulation in murine embryonic stem cells. _BMC Genomics_
10, 327 (2009). Article Google Scholar * Majumder, P.K. & Sellers, W.R. Akt-regulated pathways in prostate cancer. _Oncogene_ 24, 7465–7474 (2005). Article CAS Google Scholar * Kim,
J.B. et al. N-Cadherin extracellular repeat 4 mediates epithelial to mesenchymal transition and increased motility. _J. Cell Biol._ 151, 1193–1206 (2000). Article CAS Google Scholar *
Li, J. et al. Cardiac-specific loss of N-cadherin leads to alteration in connexins with conduction slowing and arrhythmogenesis. _Circ. Res._ 97, 474–481 (2005). Article CAS Google Scholar
* Klein, K.A. et al. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. _Nat. Med._ 3, 402–408 (1997). Article CAS Google Scholar *
Hara, T., Miyazaki, H., Lee, A., Tran, C.P. & Reiter, R.E. Androgen receptor and invasion in prostate cancer. _Cancer Res._ 68, 1128–1135 (2008). Article CAS Google Scholar * Gu, Z.
et al. Prostate stem cell antigen (PSCA) expression increases with high Gleason score, advanced stage and bone metastasis in prostate cancer. _Oncogene_ 19, 1288–1296 (2000). Article CAS
Google Scholar Download references ACKNOWLEDGEMENTS This work was supported in parts by the US National Cancer Institute Prostate Cancer SPORE at the University of California–Los Angeles
(P50CA092131-09 to R.E.R.), US Department of Defense Prostate Cancer Research grants (W81XWH-06-1-0324 to Z.A.W., W81XWH-09-1-0630 to R.E.R. and M.B.R., PC061456 to J.H.), Takeda
Pharmaceuticals, the Jean Perkins Foundation and the American Cancer Society (RSG-07-092-01-TBE to J.H.). We also thank S. and L. Resnick, the Prostate Cancer Foundation and the Luskin
Foundation for generous support and J. Said and N. Doan for immunohistochemical assessments. LNCaP-CL1 cells were provided by C.L. Tso (University of California–Los Angeles). Plasmid pΔVPR
was provided by I. Chen (University of California–Los Angeles). AUTHOR INFORMATION Author notes * Hiroshi Tanaka and Evelyn Kono: These authors contributed equally to this work. AUTHORS AND
AFFILIATIONS * Department of Urology, Geffen School of Medicine, University of California–Los Angeles, Los Angeles, California, USA Hiroshi Tanaka, Evelyn Kono, Chau P Tran, Joyce Yamashiro,
Tatsuya Shimomura, Robert Wada, Jaibin An, Matthew B Rettig & Robert E Reiter * Jonsson Comprehensive Cancer Center, Geffen School of Medicine, University of California–Los Angeles, Los
Angeles, California, USA Hiroshi Tanaka, Evelyn Kono, Chau P Tran, Joyce Yamashiro, Tatsuya Shimomura, Jiaoti Huang, Matthew B Rettig, Zev A Wainberg & Robert E Reiter * Department of
Urology, University of Tokyo, Tokyo, Japan Hideyo Miyazaki * Vancouver Prostate Center, University of British Columbia, Vancouver, British Columbia, Canada Ladan Fazli & Martin Gleave *
Department of Pathology, Geffen School of Medicine, University of California–Los Angeles, Los Angeles, California, USA Jiaoti Huang * Department of Urology, University of Washington Medical
Center, Puget Sound Veterans Health Care Administration, Seattle, Washington, USA Robert L Vessella * Department of Medicine, Veterans Affairs Greater Los Angeles Healthcare System–West Los
Angeles, Los Angeles, California, USA Jaibin An & Matthew B Rettig * Department of Human Genetics, David Geffen School of Medicine, University of California–Los Angeles, Los Angeles,
California, USA Steven Horvath * Department of Biostatistics, School of Public Health, University of California–Los Angeles, Los Angeles, California, USA Steven Horvath * Division of
Hematology and Oncology, Geffen School of Medicine, University of California–Los Angeles, Los Angeles, California, USA Zev A Wainberg * Molecular Biology Institute, Geffen School of
Medicine, University of California–Los Angeles, Los Angeles, California, USA Robert E Reiter Authors * Hiroshi Tanaka View author publications You can also search for this author inPubMed
Google Scholar * Evelyn Kono View author publications You can also search for this author inPubMed Google Scholar * Chau P Tran View author publications You can also search for this author
inPubMed Google Scholar * Hideyo Miyazaki View author publications You can also search for this author inPubMed Google Scholar * Joyce Yamashiro View author publications You can also search
for this author inPubMed Google Scholar * Tatsuya Shimomura View author publications You can also search for this author inPubMed Google Scholar * Ladan Fazli View author publications You
can also search for this author inPubMed Google Scholar * Robert Wada View author publications You can also search for this author inPubMed Google Scholar * Jiaoti Huang View author
publications You can also search for this author inPubMed Google Scholar * Robert L Vessella View author publications You can also search for this author inPubMed Google Scholar * Jaibin An
View author publications You can also search for this author inPubMed Google Scholar * Steven Horvath View author publications You can also search for this author inPubMed Google Scholar *
Martin Gleave View author publications You can also search for this author inPubMed Google Scholar * Matthew B Rettig View author publications You can also search for this author inPubMed
Google Scholar * Zev A Wainberg View author publications You can also search for this author inPubMed Google Scholar * Robert E Reiter View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS H.T. and E.K. designed and conducted _in vitro_ and _in vivo_ studies. C.P.T. generated stable N-cadherin–knockdown reagents and prepared the
manuscript. H.M. made the N-cadherin–overexpressing cell lines. J.Y. and R.W. performed gene and protein expression analyses. T.S. contributed to the _in vivo_ N-cadherin–knockdown and
antibody studies. F.L. and M.G. conducted immunohistochemical evaluation of prostate cancer specimens. J.H. contributed to immunohistochemical analyses of _in vivo_ studies. R.L.V. provided
clinical materials for the initial N-cadherin screening in metastases. J.A. and M.B.R. provided data on AKT activity. S.H. performed gene expression analysis for stem cell markers. Z.A.W.
generated the monoclonal antibodies. R.E.R. conceived of the study and supervised the project. All authors discussed the results and commented on the manuscript at all stages. CORRESPONDING
AUTHOR Correspondence to Robert E Reiter. ETHICS DECLARATIONS COMPETING INTERESTS R.E.R. has an equity interest in EMTx Therapeutics, which has an option to license and develop
N-cadherin–specific antibodies for cancer therapy. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES Supplementary Figures 1–6 (PDF 1282 kb) RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tanaka, H., Kono, E., Tran, C. _et al._ Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and
castration resistance. _Nat Med_ 16, 1414–1420 (2010). https://doi.org/10.1038/nm.2236 Download citation * Received: 16 April 2010 * Accepted: 09 September 2010 * Published: 07 November 2010
* Issue Date: December 2010 * DOI: https://doi.org/10.1038/nm.2236 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
