Taming lupus—a new understanding of pathogenesis is leading to clinical advances
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

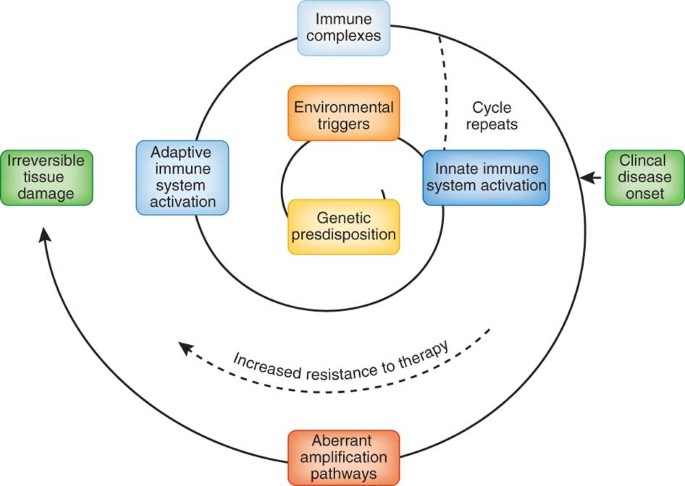
ABSTRACT Systemic lupus erythematosus (SLE) is an autoimmune disease that is characterized by the loss of tolerance to nuclear self antigens, the production of pathogenic autoantibodies and
damage to multiple organ systems. Over the years, patients with SLE have been managed largely with empiric immunosuppressive therapies, which are associated with substantial toxicities and
do not always provide adequate control of the disease. The development of targeted therapies that specifically address disease pathogenesis or progression has lagged, largely because of the
complex and heterogeneous nature of the disease, as well as difficulties in designing uniform outcome measures for clinical trials. Recent advances that could improve the treatment of SLE
include the identification of genetic variations that influence the risk of developing the disease, an enhanced understanding of innate and adaptive immune activation and regulation of
tolerance, dissection of immune cell activation and inflammatory pathways and elucidation of mechanisms and markers of tissue damage. These discoveries, together with improvements in
clinical trial design, form a platform from which to launch the development of a new generation of lupus therapies. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only
$17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS THE IMMUNOLOGY OF SYSTEMIC
LUPUS ERYTHEMATOSUS Article 15 July 2024 SYSTEMIC LUPUS ERYTHEMATOSUS GENETICS: INSIGHTS INTO PATHOGENESIS AND IMPLICATIONS FOR THERAPY Article 04 September 2024 SYSTEMIC LUPUS
ERYTHEMATOSUS: UPDATED INSIGHTS ON THE PATHOGENESIS, DIAGNOSIS, PREVENTION AND THERAPEUTICS Article Open access 17 March 2025 REFERENCES * Lateef, A. & Petri, M. Biologics in the
treatment of systemic lupus erythematosus. _Curr. Opin. Rheumatol._ 22, 504–509 (2010). PubMed Google Scholar * Navarra, S.V. et al. Efficacy and safety of belimumab in patients with
active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. _Lancet_ 377, 721–731 (2011). CAS PubMed Google Scholar * Furie, R. et al. A phase III, randomized,
placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. _Arthritis Rheum._ 63, 3918–3930 (2011).
CAS PubMed PubMed Central Google Scholar * Morel, L. et al. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. _Proc. Natl. Acad.
Sci. USA_ 97, 6670–6675 (2000). CAS PubMed Google Scholar * Lauwerys, B.R. & Wakeland, E.K. Genetics of lupus nephritis. _Lupus_ 14, 2–12 (2005). CAS PubMed Google Scholar *
Harley, J.B. et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. _Nat. Genet._ 40,
204–210 (2008). CAS PubMed PubMed Central Google Scholar * Deng, Y. & Tsao, B.P. Genetic susceptibility to systemic lupus erythematosus in the genomic era. _Nat. Rev. Rheumatol._ 6,
683–692 (2010). CAS PubMed PubMed Central Google Scholar * Flesher, D.L., Sun, X., Behrens, T.W., Graham, R.R. & Criswell, L.A. Recent advances in the genetics of systemic lupus
erythematosus. _Expert Rev. Clin. Immunol._ 6, 461–479 (2010). PubMed PubMed Central Google Scholar * Liu, K. et al. Kallikrein genes are associated with lupus and glomerular basement
membrane-specific antibody-induced nephritis in mice and humans. _J. Clin. Invest._ 119, 911–923 (2009). CAS PubMed PubMed Central Google Scholar * Sanchez, E. et al. Phenotypic
associations of genetic susceptibility loci in systemic lupus erythematosus. _Ann. Rheum. Dis._ 70, 1752–1757 (2011). CAS PubMed PubMed Central Google Scholar * Cantor, R.M., Lange, K.
& Sinsheimer, J.S. Prioritizing GWAS results: a review of statistical methods and recommendations for their application. _Am. J. Hum. Genet._ 86, 6–22 (2010). CAS PubMed PubMed Central
Google Scholar * Zhang, J. et al. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell
hyperresponsiveness. _Nat. Genet._ 43, 902–907 (2011). CAS PubMed Google Scholar * Rieck, M. et al. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. _J.
Immunol._ 179, 4704–4710 (2007). CAS PubMed Google Scholar * Taylor, K.E. et al. Risk alleles for systemic lupus erythematosus in a large case-control collection and associations with
clinical subphenotypes. _PLoS Genet._ 7, e1001311 (2011). CAS PubMed PubMed Central Google Scholar * Askanase, A.D. et al. Use of pharmacogenetics, enzymatic phenotyping, and metabolite
monitoring to guide treatment with azathioprine in patients with systemic lupus erythematosus. _J. Rheumatol._ 36, 89–95 (2009). CAS PubMed Google Scholar * Rubtsov, A.V., Rubtsova, K.,
Kappler, J.W. & Marrack, P. Genetic and hormonal factors in female-biased autoimmunity. _Autoimmun. Rev._ 9, 494–498 (2010). CAS PubMed PubMed Central Google Scholar * Smith-Bouvier,
D.L. et al. A role for sex chromosome complement in the female bias in autoimmune disease. _J. Exp. Med._ 205, 1099–1108 (2008). CAS PubMed PubMed Central Google Scholar * Ravichandran,
K.S. & Lorenz, U. Engulfment of apoptotic cells: signals for a good meal. _Nat. Rev. Immunol._ 7, 964–974 (2007). CAS PubMed Google Scholar * Marínez Valle, F., Balada, E., Ordi-Ros,
J. & Vilardell-Tarres, M. DNase 1 and systemic lupus erythematosus. _Autoimmun. Rev._ 7, 359–363 (2008). Google Scholar * Rönnblom, L. & Alm, G.V. The natural interferon-α
producing cells in systemic lupus erythematosus. _Hum. Immunol._ 63, 1181–1193 (2002). PubMed Google Scholar * Blanco, P., Palucka, A.K., Gill, M., Pascual, V. & Banchereau, J.
Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. _Science_ 294, 1540–1543 (2001). CAS PubMed Google Scholar * Bennett, L. et al. Interferon and
granulopoiesis signatures in systemic lupus erythematosus blood. _J. Exp. Med._ 197, 711–723 (2003). CAS PubMed PubMed Central Google Scholar * Bauer, J.W. et al. Elevated serum levels
of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus. _PLoS Med._ 3, e491 (2006). PubMed PubMed Central Google Scholar * Mathian, A., Weinberg,
A., Gallegos, M., Banchereau, J. & Koutouzov, S. IFN-α induces early lethal lupus in preautoimmune (New Zealand Black × New Zealand White) F1 but not in BALB/c mice. _J. Immunol._ 174,
2499–2506 (2005). CAS PubMed Google Scholar * Ramanujam, M. et al. Interferon-α treatment of female (NZW × BXSB)F(1) mice mimics some but not all features associated with the Yaa
mutation. _Arthritis Rheum._ 60, 1096–1101 (2009). CAS PubMed PubMed Central Google Scholar * Nacionales, D.C. et al. Deficiency of the type I interferon receptor protects mice from
experimental lupus. _Arthritis Rheum._ 56, 3770–3783 (2007). CAS PubMed PubMed Central Google Scholar * Agrawal, H. et al. Deficiency of type I IFN receptor in lupus-prone New Zealand
mixed 2328 mice decreases dendritic cell numbers and activation and protects from disease. _J. Immunol._ 183, 6021–6029 (2009). CAS PubMed PubMed Central Google Scholar * Banchereau, J.
& Pascual, V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. _Immunity_ 25, 383–392 (2006). CAS PubMed Google Scholar * Thacker, S.G. et al. The
detrimental effects of IFN-α on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. _J. Immunol._ 185,
4457–4469 (2010). CAS PubMed PubMed Central Google Scholar * Garcia-Romo, G.S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus
erythematosus. _Sci. Transl. Med._ 3, 73ra20 (2011). PubMed PubMed Central Google Scholar * Lande, R. et al. Neutrophils activate plasmacytoid dendritic cells by releasing
self-DNA-peptide complexes in systemic lupus erythematosus. _Sci. Transl. Med._ 3, 73ra19 (2011). PubMed PubMed Central Google Scholar * Charles, N., Hardwick, D., Daugas, E., Illei, G.G.
& Rivera, J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. _Nat. Med._ 16, 701–707 (2010). CAS PubMed PubMed Central Google Scholar *
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. _Immunity_ 34, 637–650 (2011). CAS PubMed Google Scholar * Boulé,
M.W. et al. Toll-like receptor 9–dependent and –independent dendritic cell activation by chromatin-immunoglobulin G complexes. _J. Exp. Med._ 199, 1631–1640 (2004). PubMed PubMed Central
Google Scholar * Lande, R. et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. _Nature_ 449, 564–569 (2007). CAS PubMed Google Scholar * Tian, J. et
al. Toll-like receptor 9–dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. _Nat. Immunol._ 8, 487–496 (2007). CAS PubMed Google Scholar * Gilliet, M.,
Cao, W. & Liu, Y.J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. _Nat. Rev. Immunol._ 8, 594–606 (2008). CAS PubMed Google Scholar
* Rönnblom, L. & Elkon, K.B. Cytokines as therapeutic targets in SLE. _Nat. Rev. Rheumatol._ 6, 339–347 (2010). PubMed Google Scholar * Leadbetter, E.A. et al. Chromatin–IgG complexes
activate B cells by dual engagement of IgM and Toll-like receptors. _Nature_ 416, 603–607 (2002). CAS PubMed Google Scholar * Berland, R. et al. Toll-like receptor 7–dependent loss of B
cell tolerance in pathogenic autoantibody knockin mice. _Immunity_ 25, 429–440 (2006). CAS PubMed Google Scholar * Christensen, S.R. & Shlomchik, M.J. Regulation of lupus-related
autoantibody production and clinical disease by Toll-like receptors. _Semin. Immunol._ 19, 11–23 (2007). CAS PubMed PubMed Central Google Scholar * Christensen, S.R. et al. Toll-like
receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. _Immunity_ 25, 417–428 (2006). CAS PubMed Google
Scholar * Fossati, L. et al. The Yaa gene-mediated acceleration of murine lupus: Yaa− T cells from non-autoimmune mice collaborate with Yaa+ B cells to produce lupus autoantibodies _in
vivo_. _Eur. J. Immunol._ 25, 3412–3417 (1995). CAS PubMed Google Scholar * Barbalat, R., Ewald, S.E., Mouchess, M.L. & Barton, G.M. Nucleic acid recognition by the innate immune
system. _Annu. Rev. Immunol._ 29, 185–214 (2011). CAS PubMed Google Scholar * Harley, J.B., Harley, I.T., Guthridge, J.M. & James, J.A. The curiously suspicious: a role for
Epstein-Barr virus in lupus. _Lupus_ 15, 768–777 (2006). CAS PubMed Google Scholar * Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune
response. _Nature_ 448, 501–505 (2007). CAS PubMed Google Scholar * Ishii, K.J. et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. _Nature_ 451,
725–729 (2008). CAS PubMed Google Scholar * Zhang, Z. et al. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. _Nat. Immunol._ 12, 959–965
(2011). CAS PubMed PubMed Central Google Scholar * Gall, A. et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune
disease. _Immunity_ 36, 120–131 (2012). CAS PubMed PubMed Central Google Scholar * Goodnow, C.C., Vinuesa, C.G., Randall, K.L., Mackay, F. & Brink, R. Control systems and decision
making for antibody production. _Nat. Immunol._ 11, 681–688 (2010). CAS PubMed Google Scholar * Arbuckle, M.R. et al. Development of autoantibodies before the clinical onset of systemic
lupus erythematosus. _N. Engl. J. Med._ 349, 1526–1533 (2003). CAS PubMed Google Scholar * Moulton, V.R. & Tsokos, G.C. Abnormalities of T cell signaling in systemic lupus
erythematosus. _Arthritis Res. Ther._ 13, 207 (2011). CAS PubMed PubMed Central Google Scholar * Perl, A. et al. T-cell and B-cell signaling biomarkers and treatment targets in lupus.
_Curr. Opin. Rheumatol._ 21, 454–464 (2009). CAS PubMed PubMed Central Google Scholar * Crispín, J.C., Kyttaris, V.C., Terhorst, C. & Tsokos, G.C. T cells as therapeutic targets in
SLE. _Nat. Rev. Rheumatol._ 6, 317–325 (2010). PubMed PubMed Central Google Scholar * Deng, G.M., Liu, L., Bahjat, F.R., Pine, P.R. & Tsokos, G.C. Suppression of skin and kidney
disease by inhibition of spleen tyrosine kinase in lupus-prone mice. _Arthritis Rheum._ 62, 2086–2092 (2010). CAS PubMed PubMed Central Google Scholar * Ichinose, K., Juang, Y.T.,
Crispin, J.C., Kis-Toth, K. & Tsokos, G.C. Suppression of autoimmunity and organ pathology in lupus-prone mice upon inhibition of calcium/calmodulin-dependent protein kinase type IV.
_Arthritis Rheum._ 63, 523–529 (2011). CAS PubMed PubMed Central Google Scholar * Bahjat, F.R. et al. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression
and prolongs survival in murine lupus. _Arthritis Rheum._ 58, 1433–1444 (2008). CAS PubMed Google Scholar * Linterman, M.A. et al. Follicular helper T cells are required for systemic
autoimmunity. _J. Exp. Med._ 206, 561–576 (2009). CAS PubMed PubMed Central Google Scholar * Simpson, N. et al. Expansion of circulating T cells resembling follicular helper T cells is a
fixed phenotype that identifies a subset of severe systemic lupus erythematosus. _Arthritis Rheum._ 62, 234–244 (2010). CAS PubMed Google Scholar * Odegard, J.M. et al. ICOS-dependent
extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. _J. Exp. Med._ 205, 2873–2886 (2008). CAS PubMed PubMed Central Google Scholar * Crispín, J.C. et
al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. _J. Immunol._ 181, 8761–8766 (2008). PubMed PubMed Central
Google Scholar * Wardemann, H. & Nussenzweig, M.C. B-cell self-tolerance in humans. _Adv. Immunol._ 95, 83–110 (2007). CAS PubMed Google Scholar * Liu, Z. & Davidson, A. BAFF and
selection of autoreactive B cells. _Trends Immunol._ 32, 388–394 (2011). CAS PubMed PubMed Central Google Scholar * Arechiga, A.F. et al. Cutting edge: the PTPN22 allelic variant
associated with autoimmunity impairs B cell signaling. _J. Immunol._ 182, 3343–3347 (2009). CAS PubMed PubMed Central Google Scholar * Menard, L. et al. The PTPN22 allele encoding an
R620W variant interferes with the removal of developing autoreactive B cells in humans. _J. Clin. Invest._ 121, 3635–3644 (2011). CAS PubMed PubMed Central Google Scholar * Cappione, A.
III et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. _J. Clin. Invest._ 115, 3205–3216 (2005). CAS PubMed PubMed Central Google
Scholar * Gonzalez, S.F. et al. Trafficking of B cell antigen in lymph nodes. _Annu. Rev. Immunol._ 29, 215–233 (2011). CAS PubMed Google Scholar * Kranich, J. et al. Follicular
dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. _J. Exp. Med._ 205, 1293–1302 (2008). CAS PubMed PubMed Central Google Scholar * Blair, P.A. et al.
CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. _Immunity_ 32, 129–140 (2010). CAS
PubMed Google Scholar * Campbell, D.J. & Koch, M.A. Treg cells: patrolling a dangerous neighborhood. _Nat. Med._ 17, 929–930 (2011). CAS PubMed Google Scholar * Kim, H.J. et al.
CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. _Proc. Natl. Acad. Sci. USA_ 108, 2010–2015 (2011). CAS PubMed Google
Scholar * Brownlie, R.J. et al. Distinct cell-specific control of autoimmunity and infection by FcgammaRIIb. _J. Exp. Med._ 205, 883–895 (2008). CAS PubMed PubMed Central Google Scholar
* Kim, S.J. et al. Increased IL-12 inhibits B cell differentiation to germinal center plasma cells and promotes differentiation to short-lived plasmablasts. _J. Exp. Med._ 205, 2437–2448
(2008). CAS PubMed PubMed Central Google Scholar * Herlands, R.A., William, J., Hershberg, U. & Shlomchik, M.J. Anti-chromatin antibodies drive _in vivo_ antigen-specific activation
and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. _Eur. J. Immunol._ 37, 3339–3351 (2007). CAS PubMed PubMed Central Google Scholar * Erickson, L.D. et al.
Short-circuiting long-lived humoral immunity by the heightened engagement of CD40. _J. Clin. Invest._ 109, 613–620 (2002). CAS PubMed PubMed Central Google Scholar * Cassese, G. et al.
Inflamed kidneys of NZB/W mice are a major site for the homeostasis of plasma cells. _Eur. J. Immunol._ 31, 2726–2732 (2001). CAS PubMed Google Scholar * Tokoyoda, K., Hauser, A.E.,
Nakayama, T. & Radbruch, A. Organization of immunological memory by bone marrow stroma. _Nat. Rev. Immunol._ 10, 193–200 (2010). CAS PubMed Google Scholar * Merrill, J.T. et al.
Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of
rituximab trial. _Arthritis Rheum._ 62, 222–233 (2010). CAS PubMed PubMed Central Google Scholar * Benson, M.J. et al. Cutting edge: the dependence of plasma cells and independence of
memory B cells on BAFF and APRIL. _J. Immunol._ 180, 3655–3659 (2008). CAS PubMed Google Scholar * Jacobi, A.M. et al. Effect of long-term belimumab treatment on B cells in systemic lupus
erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. _Arthritis Rheum._ 62, 201–210 (2010). CAS PubMed PubMed Central Google Scholar * Looney,
R.J., Anolik, J. & Sanz, I. A perspective on B-cell–targeting therapy for SLE. _Mod. Rheumatol._ 20, 1–10 (2010). PubMed Google Scholar * Aringer, M. et al. Current state of evidence
on “off label” therapeutic options for systemic lupus erythematosus, including biological immunosuppressive agents, in Germany, Austria, and Switzerland—a consensus report. _Lupus_ 21,
386–401 (2012). CAS PubMed Google Scholar * Hahn, B.H. Targeted therapies in systemic lupus erythematosus: successes, failures and future. _Ann. Rheum. Dis._ 70 (suppl. 1), i64–i66
(2011). CAS PubMed Google Scholar * de Laat, B., Mertens, K. & de Groot, P.G. Mechanisms of disease: antiphospholipid antibodies—from clinical association to pathologic mechanism.
_Nat. Clin. Pract. Rheumatol._ 4, 192–199 (2008). CAS PubMed Google Scholar * Lauvsnes, M.B. & Omdal, R. Systemic lupus erythematosus, the brain, and anti-NR2 antibodies. _J. Neurol._
259, 622–629 (2012). CAS PubMed Google Scholar * Faust, T.W. et al. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. _Proc. Natl. Acad. Sci. USA_
107, 18569–18574 (2010). CAS PubMed Google Scholar * DeGiorgio, L.A. et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus
erythematosus. _Nat. Med._ 7, 1189–1193 (2001). CAS PubMed Google Scholar * Matus, S. et al. Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein
causing calcium influx and apoptosis. _J. Exp. Med._ 204, 3221–3234 (2007). CAS PubMed PubMed Central Google Scholar * Kowal, C. et al. Cognition and immunity; antibody impairs memory.
_Immunity_ 21, 179–188 (2004). CAS PubMed Google Scholar * Turnberg, D. & Cook, H.T. Complement and glomerulonephritis: new insights. _Curr. Opin. Nephrol. Hypertens._ 14, 223–228
(2005). CAS PubMed Google Scholar * Bergtold, A., Gavhane, A., D'Agati, V., Madaio, M. & Clynes, R. FcR-bearing myeloid cells are responsible for triggering murine lupus
nephritis. _J. Immunol._ 177, 7287–7295 (2006). CAS PubMed Google Scholar * Anders, H.J. & Schlondorff, D. Toll-like receptors: emerging concepts in kidney disease. _Curr. Opin.
Nephrol. Hypertens._ 16, 177–183 (2007). CAS PubMed Google Scholar * Manderson, A.P., Botto, M. & Walport, M.J. The role of complement in the development of systemic lupus
erythematosus. _Annu. Rev. Immunol._ 22, 431–456 (2004). CAS PubMed Google Scholar * Woodruff, T.M., Nandakumar, K.S. & Tedesco, F. Inhibiting the C5-C5a receptor axis. _Mol.
Immunol._ 48, 1631–1642 (2011). CAS PubMed Google Scholar * Vielhauer, V., Anders, H.J. & Schlondorff, D. Chemokines and chemokine receptors as therapeutic targets in lupus nephritis.
_Semin. Nephrol._ 27, 81–97 (2007). CAS PubMed Google Scholar * Kitching, A.R. & Holdsworth, S.R. The emergence of TH17 cells as effectors of renal injury. _J. Am. Soc. Nephrol._ 22,
235–238 (2011). CAS PubMed Google Scholar * Ernandez, T. & Mayadas, T.N. Immunoregulatory role of TNFα in inflammatory kidney diseases. _Kidney Int._ 76, 262–276 (2009). CAS PubMed
Google Scholar * Bethunaickan, R. et al. A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. _J. Immunol._ 186, 4994–5003
(2011). CAS PubMed PubMed Central Google Scholar * Hill, G.S. et al. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. _Kidney Int._ 59,
304–316 (2001). CAS PubMed Google Scholar * Schlondorff, D.O. Overview of factors contributing to the pathophysiology of progressive renal disease. _Kidney Int._ 74, 860–866 (2008). CAS
PubMed Google Scholar * Deelman, L. & Sharma, K. Mechanisms of kidney fibrosis and the role of antifibrotic therapies. _Curr. Opin. Nephrol. Hypertens._ 18, 85–90 (2009). CAS PubMed
Google Scholar * Alarcón, G.S. et al. Time to renal disease and end-stage renal disease in PROFILE: a multiethnic lupus cohort. _PLoS Med._ 3, e396 (2006). PubMed PubMed Central Google
Scholar * Esdaile, J.M. et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. _Arthritis Rheum._ 44, 2331–2337
(2001). CAS PubMed Google Scholar * Symmons, D.P. & Gabriel, S.E. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. _Nat. Rev. Rheumatol._ 7, 399–408 (2011).
PubMed Google Scholar * Narshi, C.B., Giles, I.P. & Rahman, A. The endothelium: an interface between autoimmunity and atherosclerosis in systemic lupus erythematosus? _Lupus_ 20, 5–13
(2011). CAS PubMed Google Scholar * Lopez, L.R. et al. Oxidized low-density lipoprotein and β2-glycoprotein I in patients with systemic lupus erythematosus and increased carotid
intima-media thickness: implications in autoimmune-mediated atherosclerosis. _Lupus_ 15, 80–86 (2006). CAS PubMed Google Scholar * Matsuura, E., Kobayashi, K., Hurley, B.L. & Lopez,
L.R. Atherogenic oxidized low-density lipoprotein/β2-glycoprotein I (oxLDL/β2GPI) complexes in patients with systemic lupus erythematosus and antiphospholipid syndrome. _Lupus_ 15, 478–483
(2006). CAS PubMed Google Scholar * Skaggs, B.J., Hahn, B.H., Sahakian, L., Grossman, J. & McMahon, M. Dysfunctional, pro-inflammatory HDL directly upregulates monocyte PDGFRβ,
chemotaxis and TNFα production. _Clin. Immunol._ 137, 147–156 (2010). CAS PubMed PubMed Central Google Scholar * McMahon, M. et al. Dysfunctional proinflammatory high-density
lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. _Arthritis Rheum._ 60, 2428–2437 (2009). CAS PubMed PubMed Central Google Scholar *
Schanberg, L.E. et al. Use of atorvastatin in systemic lupus erythematosus in children and adolescents. _Arthritis Rheum._ 64, 285–296 (2012). CAS PubMed PubMed Central Google Scholar *
Petri, M.A., Kiani, A.N., Post, W., Christopher-Stine, L. & Magder, L.S. Lupus Atherosclerosis Prevention Study (LAPS). _Ann. Rheum. Dis._ 70, 760–765 (2011). CAS PubMed Google Scholar
* Ceribelli, A., Yao, B., Dominguez-Gutierrez, P.R. & Chan, E.K. Lupus T cells switched on by DNA hypomethylation via microRNA? _Arthritis Rheum._ 63, 1177–1181 (2011). CAS PubMed
PubMed Central Google Scholar * Pan, Y. & Sawalha, A.H. Epigenetic regulation and the pathogenesis of systemic lupus erythematosus. _Transl. Res._ 153, 4–10 (2009). CAS PubMed Google
Scholar * Dai, R. & Ahmed, S.A. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. _Transl. Res._ 157, 163–179 (2011). CAS PubMed
PubMed Central Google Scholar * Ceribelli, A. et al. MicroRNAs in systemic rheumatic diseases. _Arthritis Res. Ther._ 13, 229 (2011). CAS PubMed PubMed Central Google Scholar *
Geuking, M.B. et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. _Immunity_ 34, 794–806 (2011). CAS PubMed Google Scholar * Illei, G.G. et al.
Current state and future directions of autologous hematopoietic stem cell transplantation in systemic lupus erythematosus. _Ann. Rheum. Dis._ 70, 2071–2074 (2011). PubMed PubMed Central
Google Scholar * Choi, E.W. et al. Reversal of serological, immunological and histological dysfunction in systemic lupus erythematosus mice by long-term serial adipose tissue-derived
mesenchymal stem cell transplantation. _Arthritis Rheum._ 64, 243–253 (2012). CAS PubMed Google Scholar * Liang, J. et al. Allogenic mesenchymal stem cells transplantation in refractory
systemic lupus erythematosus: a pilot clinical study. _Ann. Rheum. Dis._ 69, 1423–1429 (2010). PubMed Google Scholar * Mok, C.C. Biomarkers for lupus nephritis: a critical appraisal. _J.
Biomed. Biotechnol._ 2010, 638413 (2010). PubMed PubMed Central Google Scholar * Bauer, J.W. et al. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease
activity: a validation study. _Arthritis Rheum._ 60, 3098–3107 (2009). CAS PubMed PubMed Central Google Scholar * Chaussabel, D. et al. A modular analysis framework for blood genomics
studies: application to systemic lupus erythematosus. _Immunity_ 29, 150–164 (2008). CAS PubMed PubMed Central Google Scholar * Hinze, C.H. et al. Neutrophil gelatinase-associated
lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity. _Arthritis Rheum._ 60, 2772–2781 (2009). PubMed PubMed Central
Google Scholar * Rovin, B.H. et al. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. _J. Am. Soc. Nephrol._ 16, 467–473 (2005). CAS PubMed Google Scholar *
Rubinstein, T. et al. Urinary neutrophil gelatinase-associated lipocalin as a novel biomarker for disease activity in lupus nephritis. _Rheumatology (Oxford)_ 49, 960–971 (2010). CAS Google
Scholar * Guiducci, C. et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. _Nature_ 465, 937–941 (2010). CAS PubMed PubMed Central Google Scholar *
Yuan, W., DiMartino, S.J., Redecha, P.B., Ivashkiv, L.B. & Salmon, J.E. Systemic lupus erythematosus monocytes are less responsive to interleukin-10 in the presence of immune complexes.
_Arthritis Rheum._ 63, 212–218 (2011). CAS PubMed PubMed Central Google Scholar * Ramanujam, M. & Davidson, A. Targeting of the immune system in systemic lupus erythematosus. _Expert
Rev. Mol. Med._ 10, e2 (2008). PubMed Google Scholar * Wofsy, D.S., Shropshire, S.M., Hillson, J.L. & Diamond, B. Abatacept for lupus nephritis: alternative outcome measures support
opposing interpretations of data From a multicenter, randomized, double-blind, placebo-controlled phase II/III study. ACR presentation, number 2474 (8 November 2011). * Lenert, P.S.
Classification, mechanisms of action, and therapeutic applications of inhibitory oligonucleotides for Toll-like receptors (TLR) 7 and 9. _Mediators Inflamm._ 2010, 986596 (2010). PubMed
PubMed Central Google Scholar * Kyttaris, V.C. & Tsokos, G.C. Targeting lymphocyte signaling pathways as a therapeutic approach to systemic lupus erythematosus. _Curr. Opin.
Rheumatol._ 23, 449–453 (2011). CAS PubMed PubMed Central Google Scholar * Schiffer, L. et al. Short term administration of costimulatory blockade and cyclophosphamide induces remission
of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. _J. Immunol._ 171, 489–497 (2003). CAS PubMed Google Scholar *
Daikh, D.I. & Wofsy, D. Cutting edge: reversal of murine lupus nephritis with CTLA4Ig and cyclophosphamide. _J. Immunol._ 166, 2913–2916 (2001). CAS PubMed Google Scholar * Ng, K.P.
et al. B cell depletion therapy in systemic lupus erythematosus: long-term follow-up and predictors of response. _Ann. Rheum. Dis._ 66, 1259–1262 (2007). CAS PubMed PubMed Central Google
Scholar * Bloom, O. et al. Generation of a unique small molecule peptidomimetic that neutralizes lupus autoantibody activity. _Proc. Natl. Acad. Sci. USA_ 108, 10255–10259 (2011). CAS
PubMed Google Scholar * Clynes, R., Dumitru, C. & Ravetch, J.V. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. _Science_ 279, 1052–1054
(1998). CAS PubMed Google Scholar * Gambaro, G. & Kong, N.C. Glycosaminoglycan treatment in glomerulonephritis? An interesting option to investigate. _J. Nephrol._ 23, 244–252 (2010).
PubMed Google Scholar * Nguyen, T.Q. & Goldschmeding, R. Bone morphogenetic protein-7 and connective tissue growth factor: novel targets for treatment of renal fibrosis? _Pharm. Res._
25, 2416–2426 (2008). CAS PubMed Google Scholar * Renner, B. et al. Binding of factor H to tubular epithelial cells limits interstitial complement activation in ischemic injury. _Kidney
Int._ 80, 165–173 (2011). CAS PubMed PubMed Central Google Scholar * Tse, K.C. et al. Angiotensin inhibition or blockade for the treatment of patients with quiescent lupus nephritis and
persistent proteinuria. _Lupus_ 14, 947–952 (2005). CAS PubMed Google Scholar * McMahon, M., Hahn, B.H. & Skaggs, B.J. Systemic lupus erythematosus and cardiovascular disease:
prediction and potential for therapeutic intervention. _Expert Rev. Clin. Immunol._ 7, 227–241 (2011). PubMed PubMed Central Google Scholar * Stohl, W. et al. Belimumab reduces
autoantibodies, normalizes low complement, and reduces select B-cell populations in patients with systemic lupus erythematosus. _Arthritis Rheum._ published online doi:10.1002/art.34400 (24
January 2012). * Liu, Z. & Davidson, A. BAFF inhibition: a new class of drugs for the treatment of autoimmunity. _Exp. Cell Res._ 317, 1270–1277 (2011). CAS PubMed PubMed Central
Google Scholar * Mackay, F. & Schneider, P. Cracking the BAFF code. _Nat. Rev. Immunol._ 9, 491–502 (2009). CAS PubMed Google Scholar * Stohl, W. et al. Belimumab reduces
autoantibodies, normalizes low complement, and reduces select B-cell populations in patients with systemic lupus erythematosus. _Arthritis Rheum._ published online doi:10.1002/art.34400 (24
January 2012). Download references ACKNOWLEDGEMENTS This work was supported by US National Institutes of Health grants R01 DK085241-01, R01 AI083901 and R21 AR057930. The authors thank T.
Rothstein and A. Boneparth for critical reading of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Center for Autoimmunity and Musculoskeletal Diseases, Feinstein Institute for
Medical Research, Manhasset, New York, USA Zheng Liu & Anne Davidson Authors * Zheng Liu View author publications You can also search for this author inPubMed Google Scholar * Anne
Davidson View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Anne Davidson. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no competing financial interests. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Liu, Z., Davidson, A. Taming lupus—a new
understanding of pathogenesis is leading to clinical advances. _Nat Med_ 18, 871–882 (2012). https://doi.org/10.1038/nm.2752 Download citation * Published: 06 June 2012 * Issue Date: June
2012 * DOI: https://doi.org/10.1038/nm.2752 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
