Accelerated transport and maturation of lysosomal α–galactosidase a in fabry lymphoblasts by an enzyme inhibitor
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT FABRY DISEASE IS A DISORDER OF GLYCOSPHINGOLIPID METABOLISM CAUSED BY DEFICIENCY OF LYSOSOMAL Α–GALACTOSIDASE A (Α–GAL A), RESULTING IN RENAL FAILURE ALONG WITH PREMATURE MYOCARDIAL
INFARCTION AND STROKES1,2. NO EFFECTIVE TREATMENT OF THIS DISORDER IS AVAILABLE AT PRESENT. STUDIES OF RESIDUAL ACTIVITIES OF MUTANT ENZYMES IN MANY FABRY PATIENTS SHOWED THAT SOME OF THEM
HAD KINETIC PROPERTIES SIMILAR TO THOSE FOR NORMAL Α–GAL A, BUT WERE SIGNIFICANTLY LESS STABLE, ESPECIALLY IN CONDITIONS OF NEUTRAL PH (REFS. 3,4,5 ). THE BIOSYNTHETIC PROCESSING WAS DELAYED
IN CULTURED FIBROBLASTS OF A FABRY PATIENT6, AND THE MUTANT PROTEIN FORMED AN AGGREGATE IN ENDOPLASMIC RETICULUM7, INDICATING THAT THE ENZYME DEFICIENCY IN SOME MUTANTS WAS MAINLY CAUSED BY
ABORTIVE EXIT FROM THE ENDOPLASMIC RETICULUM, LEADING TO EXCESSIVE DEGRADATION OF THE ENZYME. WE REPORT HERE THAT 1–DEOXY–GALACTONOJIRIMYCIN (DGJ), A POTENT COMPETITIVE INHIBITOR OF Α–GAL
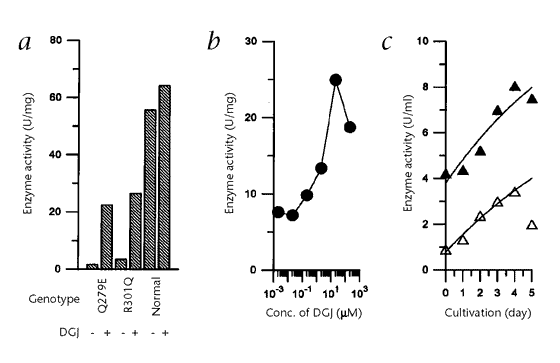
A, EFFECTIVELY ENHANCED Α–GAL A ACTIVITY IN FABRY LYMPHOBLASTS, WHEN ADMINISTRATED AT CONCENTRATIONS LOWER THAN THAT USUALLY REQUIRED FOR INTRACELLULAR INHIBITION OF THE ENZYME. DGJ SEEMED
TO ACCELERATE TRANSPORT AND MATURATION OF THE MUTANT ENZYME. ORAL ADMINISTRATION OF DGJ TO TRANSGENIC MICE OVEREXPRESSING A MUTANT Α–GAL A SUBSTANTIALLY ELEVATED THE ENZYME ACTIVITY IN SOME
ORGANS. WE PROPOSE A NEW MOLECULAR THERAPEUTIC STRATEGY FOR GENETIC METABOLIC DISEASES OF ADMINISTERING COMPETITIVE INHIBITORS AS 'CHEMICAL CHAPERONS' AT SUB–INHIBITORY
INTRACELLULAR CONCENTRATIONS. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access
to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read
our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS OPTIMIZING HUMAN Α-GALACTOSIDASE FOR TREATMENT OF FABRY DISEASE Article Open access 23 March 2023 THERAPEUTIC
EFFECTS OF LOMERIZINE ON VASCULOPATHY IN FABRY DISEASE Article Open access 02 April 2025 PRECLINICAL EVALUATION OF FLT190, A LIVER-DIRECTED AAV GENE THERAPY FOR FABRY DISEASE Article Open
access 11 January 2023 REFERENCES * Brady, R.O. _ et al._ Enzymatic defect in Fabry's disease. _N. Engl. J. Med._ 276, 1163–1167 ( 1967). Article CAS Google Scholar * Desnick, R.J.,
Ioannou, Y.A. & Eng, C.M. in _The Metabolic and Molecular Bases of Inherited Disease_ (eds. Scriver, C.R., Beaudet, A.L., Sly, W.S. & Valle, D.) 2741–2784 (McGraw–Hill, New York,
1995). Google Scholar * Romeo, G. _et al._ Residual activity of α–galactosidase A in Fabry's disease. _Biochem. Genet._ 13, 615– 628 (1975). Article CAS Google Scholar * Bishop,
D.F., Grabowski, G.A. & Desnick, R.J. Fabry disease: An asymptomatic hemizygote with significant residual α–galactosidase A activity. _Am. J. Hum. Genet. _ 33, 71A (1981). Google Scholar
* Ishii, S., Kase, R., Sakuraba, H. & Suzuki, Y. Characterization of a mutant α–galactosidase gene product for the late–onset cardiac form of Fabry disease. _Biochem. Biophys. Res.
Comm._ 197, 1585–1589 (1993). Article CAS Google Scholar * Lemansky, P. _ et al._ Synthesis and processing of α–galactosidase A in human fibroblasts. _J. Biol. Chem._ 262, 2062–2065
(1987). CAS PubMed Google Scholar * Ishii, S. _et al._ Aggregation of the inactive form of human α–galactosidase in the endoplasmic reticulum. _Biochem. Biophys. Res. Comm._ 220, 812–815
(1996). Article CAS Google Scholar * Sakuraba, H. _ et al._ Identification of point mutations in the α–galactosidase A gene in classical and atypical hemizygotes with Fabry disease. _ Am.
J. Hum. Genet._ 47, 784–789 (1990). CAS PubMed PubMed Central Google Scholar * Ishii, S., Sakuraba, H. & Suzuki, Y. Point mutations in the upstream region of the α–galactosidase A
gene exon 6 in an atypical variant of Fabry disease. _Hum. Genet. _ 89, 29–32 ( 1992). Article CAS Google Scholar * Ishii, S. _et al._ α–Galactosidase transgenic mouse: Heterogenous gene
expression and posttranslational glycosylation in tissues. _Glycoconj. J._ 15, 591–594 ( 1998). Article CAS Google Scholar * Gilliland, G., Perrin, S., Blanchard, K. & Bunn, H.F.
Analysis of cytokine mRNA and DNA: Detection and quantitation by competitive polymerase chain reaction. _Proc. Natl. Acad. Sci. USA_ 87, 2725–2729 (1990). Article CAS Google Scholar *
Fleischer, S. & Kervina, M. Subcellular fractionation of rat liver. _Methods Enzymol._ 31, 6– 41 (1974). Article CAS Google Scholar * Hurtley, S.M. & Helenius, A. Protein
oligomerization in the endoplasmic reticulum. _Annu. Rev. Cell Biol._ 5, 277–307 (1989). Article CAS Google Scholar * Dale, M. P. _ et al._ Reversible inhibitors of β–glucosidase. _
Biochemistry_ 24, 3530–3539 (1985). Article CAS Google Scholar * Asano, N., Oseki, K., Kizu, H. & Matsui, K. Nitrogen–in–the–ring pyranoses and furanoses: Structural basis of
inhibition of mammalian glycosidases. _J. Med. Chem._ 37, 3701– 3706 (1994). Article CAS Google Scholar * Asano, N. _et al._ Homonojirimycin isomers and glycosides from _Aglaonema treubii
_. _J. Nat. Prod._ 60, 98– 101 (1997). Article CAS Google Scholar * Block, T.M. _ et al._ Treatment of chronic hepadnavirus infection in a woodchuck animal model with an inhibitor of
protein folding and trafficking. _ Nature Med._ 4, 610–614 ( 1998). Article CAS Google Scholar * Goss, P.E. _ et al._ A phase I study of swainsonine in patients with advanced
malignancies. _Cancer Res._ 54, 1450–1457 (1994). CAS PubMed Google Scholar * Abe, A., Radin, N. S. & Shayman, J.A. Induction of glucosylceramide synthase by synthase inhibitors and
ceramide. _Biochim. Biophys. Acta_ 1299 , 333–341 (1996). Article Google Scholar * Neuenhofer, S., Schwarzmann, G., Egge, H. & Sandhoff, K. Synthesis of lysogangliosides,
_Biochemistry_ 24, 525–532 (1985). Article CAS Google Scholar * Mitsutake, S., Kita, K., Okino, N. & Ito, M. [14C]–Ceramide synthesis by sphingolipid ceramide _N_–deacylase: New assay
for ceramidase activity detection. _Anal. Biochem._ 247, 52–57 (1997). Article CAS Google Scholar * Oshima, A. _ et al._ Intracellular processing and maturation of mutant gene products
in hereditary β–galactosidase deficiency (β–galactosidosis). _Hum. Genet._ 93, 109–114 (1994). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS _We thank Y. Kushi and S.
Handa (Tokyo Medical and Dental University) for providing CTH; M. Ito (Kyushu University) for providing sphingolipid ceramide N–deacylase; K. Mannen (Oita Medical University) for animal
care; A. Oshima (National Institute of Infectious Diseases, Japan), N. Ishida, H. Kubo, and Y. Hashimoto (The Tokyo Metropolitan Institute of Medical Science) for technical advice; R.O.
Brady (NIH) for discussions; Y.C. Lee (The Johns Hopkins University) for critical reading and advice on preparing the manuscript as well as suggestions; and A. Suzuki (The Tokyo Metropolitan
Institute of Medical Science) for discussions. This work was supported in part by grants from the Ministry of Education, Science, Sports and Culture and the Ministry of Health and Welfare
of Japan._ AUTHOR INFORMATION Author notes * Jian-Qiang Fan Present address: Department of Human Genetics, Mt Sinai School of Medicine, Fifth Avenue at 100th Street, New York, New York,
10029, USA AUTHORS AND AFFILIATIONS * Department of Membrane Biochemistry, The Tokyo Metropolitan Institute of Medical Science, Tokyo, 113–8613, Japan Jian-Qiang Fan * The Tokyo Metropolitan
Institute of Medical Science, , Tokyo, 113–8613, Japan Yoshiyuki Suzuki * Usuki Bio Research Center, Oita, 875–0061, Japan Satoshi Ishii * Faculty of Pharmaceutical Sciences, Hokuriku
University , Kanazawa, 920–1181, Japan Naoki Asano Authors * Jian-Qiang Fan View author publications You can also search for this author inPubMed Google Scholar * Satoshi Ishii View author
publications You can also search for this author inPubMed Google Scholar * Naoki Asano View author publications You can also search for this author inPubMed Google Scholar * Yoshiyuki Suzuki
View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Jian-Qiang Fan. RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Fan, JQ., Ishii, S., Asano, N. _et al._ Accelerated transport and maturation of lysosomal α–galactosidase A in Fabry lymphoblasts by an enzyme inhibitor.
_Nat Med_ 5, 112–115 (1999). https://doi.org/10.1038/4801 Download citation * Received: 28 September 1998 * Accepted: 03 November 1998 * Issue Date: January 1999 * DOI:
https://doi.org/10.1038/4801 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
