Long-term correction of canine hemophilia b by gene transfer of blood coagulation factor ix mediated by adeno-associated viral vector
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT HEMOPHILIA B IS A SEVERE X-LINKED BLEEDING DIATHESIS CAUSED BY THE ABSENCE OF FUNCTIONAL BLOOD COAGULATION FACTOR IX, AND IS AN EXCELLENT CANDIDATE FOR TREATMENT OF A GENETIC
DISEASE BY GENE THERAPY. USING AN ADENO-ASSOCIATED VIRAL VECTOR, WE DEMONSTRATE SUSTAINED EXPRESSION (>17 MONTHS) OF FACTOR IX IN A LARGE-ANIMAL MODEL AT LEVELS THAT WOULD HAVE A
THERAPEUTIC EFFECT IN HUMANS (UP TO 70 NG/ML, ADEQUATE TO ACHIEVE PHENOTYPIC CORRECTION, IN AN ANIMAL INJECTED WITH 8.5 × 10 12 VECTOR PARTICLES/KG). THE FIVE HEMOPHILIA B DOGS TREATED
SHOWED STABLE, VECTOR DOSE-DEPENDENT PARTIAL CORRECTION OF THE WHOLE BLOOD CLOTTING TIME AND, AT HIGHER DOSES, OF THE ACTIVATED PARTIAL THROMBOPLASTIN TIME. IN CONTRAST TO OTHER VIRAL GENE
DELIVERY SYSTEMS, THIS MINIMALLY INVASIVE PROCEDURE, CONSISTING OF A SERIES OF PERCUTANEOUS INTRAMUSCULAR INJECTIONS AT A SINGLE TIMEPOINT, WAS NOT ASSOCIATED WITH LOCAL OR SYSTEMIC
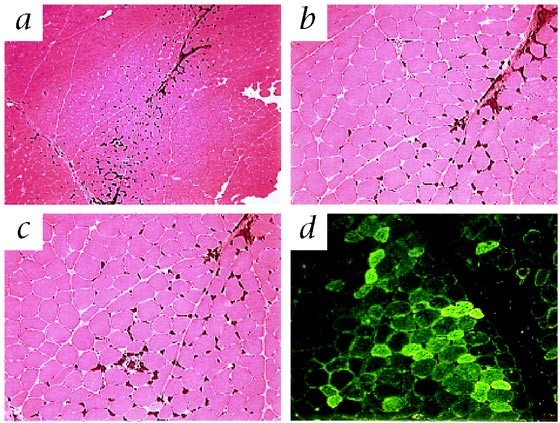
TOXICITY. EFFICIENT GENE TRANSFER TO MUSCLE WAS SHOWN BY IMMUNOFLUORESCENCE STAINING AND DNA ANALYSIS OF BIOPSIED TISSUE. IMMUNE RESPONSES AGAINST FACTOR IX WERE EITHER ABSENT OR TRANSIENT.
THESE DATA PROVIDE STRONG SUPPORT FOR THE FEASIBILITY OF THE APPROACH FOR THERAPY OF HUMAN SUBJECTS. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS A LONG-TERM STUDY OF AAV GENE THERAPY IN
DOGS WITH HEMOPHILIA A IDENTIFIES CLONAL EXPANSIONS OF TRANSDUCED LIVER CELLS Article 16 November 2020 LIVER-DIRECTED LENTIVIRAL GENE THERAPY CORRECTS HEMOPHILIA A MICE AND ACHIEVES
NORMAL-RANGE FACTOR VIII ACTIVITY IN NON-HUMAN PRIMATES Article Open access 04 May 2022 PRE-CLINICAL EVALUATION OF AN ENHANCED-FUNCTION FACTOR VIII VARIANT FOR DURABLE HEMOPHILIA A GENE
THERAPY IN MALE MICE Article Open access 21 August 2024 REFERENCES * Lofqvist, T., Nilsson, I.M., Berntorp, E. & Pettersson, H. Haemophilia prophylaxis in young patients—a long term
follow up. _ J. Int. Med._ 241, 395–400 (1997). Article CAS Google Scholar * Eyster, M.E. _ et al_. Central nervous system bleeding in hemophiliacs. _ Blood_ 51, 1179–1188 ( 1978). CAS
PubMed Google Scholar * Bray, G.L. & Lubahn, N.L. Hemophilia presenting with–intracranial hemorrhage. An approach to the infant with intracranial bleeding and coagulopathy. _Am. J.
Dis. Children_ 141, 1215– 1217 (1987). Article CAS Google Scholar * Lusher, J.M. Prophylaxis in children with hemophilia: Is it the optimal treatment? _ Thromb. Haemost._ 78, 726–729
(1997). Article CAS PubMed Google Scholar * Ragni, M.V., Hord, J.D. & Blatt, J. Central venous catheter infection in hemophiliacs undergoing prophylaxis or immune tolerance with
clotting factor concentrate. _ Haemophilia_ 3, 90–95 ( 1997). Article CAS PubMed Google Scholar * Kay, M.A. _et al_. _In vivo_ gene therapy of hemophilia B: Sustained partial correction
in factor IX-deficient dogs. _Science_ 262 , 117–119 (1993). Article CAS PubMed Google Scholar * Herzog, R.W. _ et al_. Stable gene transfer and expression of human blood coagulation
factor IX after intramuscular injection of recombinant adeno-associated virus. _Proc. Nat. Acad. Sci. USA_ 94, 5804– 5809 (1997). Article CAS PubMed PubMed Central Google Scholar *
Xiao, X., Li, J. & Samulski, R.J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. _J. Virol. _ 70, 8098–8108 ( 1996). CAS
PubMed PubMed Central Google Scholar * Monahan P.E. _ et al_. Direct intramuscular injection with recombinant–AAV vectors results in sustained expression in a dog model of hemophilia. _
Gene Ther._ 5, 40–49 ( 1998). Article CAS PubMed Google Scholar * Fisher, K.J. _ et al_. Recombinant adeno-associated virus for muscle directed gene therapy. _Nature Med._ 3:306– 312
(1997). Article CAS PubMed Google Scholar * Yao, S.-N., Smith, K.J. & Kurachi, K. Primary myoblast-mediated gene transfer: Persistent expression of human factor IX in mice. _Gene
Ther._ 1 , 99–107 (1994). CAS PubMed Google Scholar * Verma, I.M. & Somia, N. Gene therapy - promises, problems and prospects. _Nature_ 389, 239– 242 (1997). Article CAS PubMed
Google Scholar * Kay, M.A. _et al_. _In vivo_ hepatic gene therapy: Complete albeit transient correction of factor IX deficiency in hemophilia B dogs. _Proc. Natl. Acad. Sci. USA_ 91,
2353–2357 (1994). Article CAS PubMed PubMed Central Google Scholar * Fang, B. _et al_. Lack of persistence of E1– recombinant adenoviral vectors containing a temperature-sensitive E2A
mutation in immunocompetent mice and hemophilia B dogs. _Gene Ther._ 3, 217–222 (1996). CAS PubMed Google Scholar * Kohn, D.B. _ et al._ T lymphocytes with a normal ADA gene accumulate
after transplantation of transduced autologous umbilical cord blood CD34+ cells in ADA-deficient SCID neonates. _Nature Med._ 4, 775–780 (1998). Article CAS PubMed Google Scholar *
Nienhuis, A.W., McDonagh, K.T. & Bodine, D.M. Gene transfer into hematopoietic stem cells. _ Cancer_ 67, 2700–2704 ( 1991). Article CAS PubMed Google Scholar * Matsushita, T. _ et
al_. Adeno-associated virus vectors can be efficiently produced without helper virus. _Gene Ther._ 5, 938– 945 (1998). Article CAS PubMed Google Scholar * Evans, J.P., Brinkhous, K.M.,
Brayer, G.D., Reisner. H.W. & High, K.A. Canine hemophilia B resulting from a point mutation with unusual consequences. _Proc. Natl. Acad. Sci. USA_ 86, 10095–10099 (1989). Article CAS
PubMed PubMed Central Google Scholar * Mitruka, B.M., Rawnsley, H.M. & Vadehra, D.V. in _Animals for Medical Research–Models for the Study of Human Disease_. 216 (John Wiley &
Sons, New York, 1976). Google Scholar * Nakai, H. _et al_. Adeno-associated viral vector-mediated gene transfer of human blood coagulation factor IX into mouse liver. _Blood_ 91, 4600–4607
(1998). CAS PubMed Google Scholar * Baskar, J.F. _ et al_. The enhancer domain of the human cytomegalovirus major immediate-early promoter determines cell type-specific expression in
transgenic mice. _ J. Virol._ 70, 3207 (1996). CAS PubMed PubMed Central Google Scholar * Kay, M.A. _et al_. Expression of human alpha1-antitrypsin in dogs after autologous
trannsplantation of retroviral transduced hepatocytes. _Proc. Natl. Acad. Sci. USA_ 89, 89–93 (1991). Article Google Scholar * Svensson, E.C. _ et al_. Long-term erythropoietin expression
in rodents and non-human primates following intramuscular injection of a replication-defective adenoviral vector. _Hum. Gene Ther._ 8, 1797– 1806 (1997). Article CAS PubMed Google Scholar
* Yang, Y., Haecker, S.E., Su, Q. & Wilson JM. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. _Hum. Molec. Genet._ 5, 1703–1712 (1996). Article CAS
PubMed Google Scholar * Dai. Y. et al. Cellular and humoral responses to adenoviral vectors containing factor IX gene: Tolerization of factor IX and vector antigens allows for long-term
expression. _Proc. Natl. Acad. Sci. USA_ 92, 1401–1405 (1995). Article CAS PubMed PubMed Central Google Scholar * Jooss, K., Yang, Y., Fisher, K.J. & Wilson, J.M. Transduction of
dentritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. _J. Virol._ 72, 4212–4223 (1998). CAS PubMed PubMed Central Google Scholar *
Xiao, X., Li, J. & Samulski, R.J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. _J. Virol._ 72, 2224–2232 (1998). CAS PubMed
PubMed Central Google Scholar * Ferrari, F.K., Xiao, X., McCarty, D. & Samulski, R.J. New developments in the generation of Ad-free, high titer rAAV gene therapy vectors. _ Nature
Med._ 3, 1295–1297 (1997). Article CAS PubMed Google Scholar * High, K.A. Factor IX: Molecular structure, epitopes, and mutations associated with inhibitor formation. _Adv. Exp. Med.
Biol._ 386, 79– 86 (1995). Article CAS PubMed Google Scholar * Bray, G.L. _ et al_. A Multicenter study of recombinant factor VIII (Recombinate): Safety, efficacy, and inhibitor risk in
previously untreated patients with hemophilia A. _Blood_ 83, 2428– 2435 (1994). CAS PubMed Google Scholar * Lusher, J.M., Arkin, S., Abildgaard, C.F. & Schwartz. Recombinant factor
VIII for the treatment of previously untreated patients with hemophilia A. _N. Engl. J. Med._ 328, 453–459 ( 1993). Article CAS PubMed Google Scholar * Gilles, J.G.G., Arnout, J.,
Vermylen, J. & Saint-Remy, J.-M.R. Anti-factor VIII antibodies of hemophiliac patients are frequently directed towards nonfunctional determinants and do not exhibit isotypic restriction.
_Blood_ 82, 2452–2461 (1993). CAS PubMed Google Scholar * Gilles, J.G., Desqueper, B., Lenk, H., Vermylen, J. & Saint-Remy, J.M. Neutralizing anti-idiotypic antibodies to factor VIII
inhibitors after desensitization in patients with hemophilia A. _ J. Clin. Invest._ 97: 1382–1388 (1996). Article CAS PubMed PubMed Central Google Scholar * Miao, C.H. _ et al_. The
kinetics of rAAV integration in the liver. _ Nat. Genet._ 19, 13–15 ( 1998). Article CAS PubMed Google Scholar * Duan, D. _et al_. Circular intermediates of recombinant adeno-associated
virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. _J. Virol._ 72, 8568–8577 (1998). CAS PubMed PubMed Central Google Scholar
* Afione, S.A. _ et al_. _In vivo_ model of adeno-associated virus vector persistence and rescue. _J Virol_ 70, 3235– 3241 (1996). CAS PubMed PubMed Central Google Scholar * Evans, J.P.,
Watzke, H.H., Ware, J.L., Stafford, D.W. & High, K.A. Molecular cloning of a cDNA encoding canine factor IX. _Blood_ 74, 207–212 (1989). CAS PubMed Google Scholar * Kessler, P.D. _
et al_. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. _Proc. Natl. Acad. Sci. USA_ 93, 14082–14087 (1996). Article CAS
PubMed PubMed Central Google Scholar * Brinkhous K.M., Landen, C.N. & Read, M.S. Evaluation of sensitivity of whole blood clotting time (WBCT), partial thromboplastin time (PTT), and
F.IX one-stage bioassay tests with low plasma F.IX levels observed with transfusion or gene therapy in canine hemophilia B. _Blood_ 82, Suppl.1, 592a (1993). Google Scholar * Walter, J.,
You, Q., Hagstrom, N., Sands, M. & High, K.A. Successful expression of human factor IX following repeat administration of an adenoviral vector in mice. _Proc. Natl. Acad. Sci. USA_ 93,
3056–3061 ( 1996). Article CAS PubMed PubMed Central Google Scholar * Kung, S.-H. _ et al_. Human factor IX corrects the bleeding diathesis of mice with hemophilia B. _Blood_ 91, 784–
790 (1998). CAS PubMed Google Scholar * Sambrook, J., Fritsch, E.F. & Maniatis, T. in _Molecular Cloning_ (ed. Nolan, C.) 9.16 –9.19 (Cold Spring Harbor Laboratory Press, Cold Spring
Harbor, New York,1989). Google Scholar Download references ACKNOWLEDGEMENTS The authors thank Acuson for supplying the ultrasound equipment, M. Haskins for making the normal dog experiment
possible, and J.H. Liu, S.J. Tai, and M.L. McCleland, as well as the Cell Morphology Core of the Institute for Human Gene Therapy at the University of Pennsylvania, for technical assistance.
We also acknowledge the work of the staff at the vector production facility at Avigen, and the staff of the Francis Owen Blood Research Laboratory at the University of North Carolina-Chapel
Hill. This work was supported by National Institutes of Health Grants R01 HL53668 and P50 HL54500 to K.A.H., and Avigen, a company in which K.A.H. holds equity. P.A.F. is supported by the
Katherine Dormandy Trust for Hemophilia, and J.H.H. was supported by NIH grant F32 HL09397 AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Departments of Pediatrics and Pathology, University
of Pennsylvania Medical Center and The Children's Hospital of Philadelphia, Philadelphia, 19104, Pennsylvania, USA Roland W. Herzog, J. Nathan Hagstrom, Paul A. Fields & Katherine
A. High * Department of Surgery, The Children's Hospital of Philadelphia, Philadelphia, 19104, Pennsylvania, USA Edmund Y. Yang * Avigen Inc., 1201 Harbor Bay Parkway #1000, Alameda,
94502, California, USA Linda B. Couto, Melissa Burton, Gregory M. Podsakoff & Gary J. Kurtzman * Department of Pathology and Laboratory Medicine, University of North Carolina at Chapel
Hill, Chapel Hill, 27599, North Carolina, USA Dan Elwell, Dwight A. Bellinger, Marjorie S. Read, Kenneth M. Brinkhous & Timothy C. Nichols * Institute for Human Gene Therapy, University
of Pennsylvania , Philadelphia, 19104, Pennsylvania, USA Katherine A. High Authors * Roland W. Herzog View author publications You can also search for this author inPubMed Google Scholar *
Edmund Y. Yang View author publications You can also search for this author inPubMed Google Scholar * Linda B. Couto View author publications You can also search for this author inPubMed
Google Scholar * J. Nathan Hagstrom View author publications You can also search for this author inPubMed Google Scholar * Dan Elwell View author publications You can also search for this
author inPubMed Google Scholar * Paul A. Fields View author publications You can also search for this author inPubMed Google Scholar * Melissa Burton View author publications You can also
search for this author inPubMed Google Scholar * Dwight A. Bellinger View author publications You can also search for this author inPubMed Google Scholar * Marjorie S. Read View author
publications You can also search for this author inPubMed Google Scholar * Kenneth M. Brinkhous View author publications You can also search for this author inPubMed Google Scholar * Gregory
M. Podsakoff View author publications You can also search for this author inPubMed Google Scholar * Timothy C. Nichols View author publications You can also search for this author inPubMed
Google Scholar * Gary J. Kurtzman View author publications You can also search for this author inPubMed Google Scholar * Katherine A. High View author publications You can also search for
this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Katherine A. High. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Herzog, R.,
Yang, E., Couto, L. _et al._ Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. _Nat Med_ 5, 56–63
(1999). https://doi.org/10.1038/4743 Download citation * Received: 17 September 1998 * Accepted: 30 November 1998 * Issue Date: January 1999 * DOI: https://doi.org/10.1038/4743 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative
