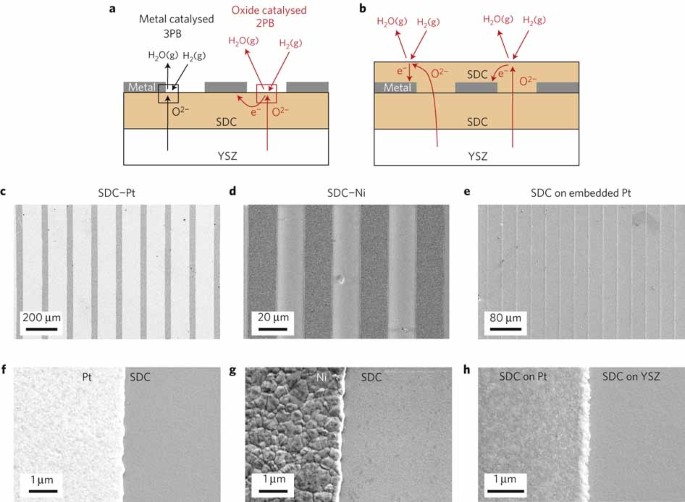
High electrochemical activity of the oxide phase in model ceria–pt and ceria–ni composite anodes
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Fuel cells, and in particular solid-oxide fuel cells (SOFCs), enable high-efficiency conversion of chemical fuels into useful electrical energy and, as such, are expected to play a
major role in a sustainable-energy future. A key step in the fuel-cell energy-conversion process is the electro-oxidation of the fuel at the anode. There has been increasing evidence in
recent years that the presence of CeO2-based oxides (ceria) in the anodes of SOFCs with oxygen-ion-conducting electrolytes significantly lowers the activation overpotential for hydrogen
oxidation. Most of these studies, however, employ porous, composite electrode structures with ill-defined geometry and uncontrolled interfacial properties. Accordingly, the means by which
electrocatalysis is enhanced has remained unclear. Here we demonstrate unambiguously, through the use of ceria–metal structures with well-defined geometries and interfaces, that the
near-equilibrium H2 oxidation reaction pathway is dominated by electrocatalysis at the oxide/gas interface with minimal contributions from the oxide/metal/gas triple-phase boundaries, even
for structures with reaction-site densities approaching those of commercial SOFCs. This insight points towards ceria nanostructuring as a route to enhanced activity, rather than the
traditional paradigm of metal-catalyst nanostructuring. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS
Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ELECTROCHEMICAL EVALUATION OF POROUS CAFE2O4 ANODE MATERIAL PREPARED VIA
SOLUTION COMBUSTION SYNTHESIS AT INCREASING FUEL-TO-OXIDIZER RATIOS AND CALCINATION TEMPERATURES Article Open access 23 February 2022 PERFORMANCE ENHANCEMENT AND DEGRADATION MECHANISM
IDENTIFICATION OF A SINGLE-ATOM CO–N–C CATALYST FOR PROTON EXCHANGE MEMBRANE FUEL CELLS Article 30 November 2020 NANOENGINEERING OF CATHODE LAYERS FOR SOLID OXIDE FUEL CELLS TO ACHIEVE
SUPERIOR POWER DENSITIES Article Open access 25 June 2021 REFERENCES * Jiang, S. P. & Chan, S. H. A review of anode materials development in solid oxide fuel cells. _J. Mater. Sci._ 39,
4405–4439 (2004). Article CAS Google Scholar * Sholklapper, T. Z., Kurokawa, H., Jacobson, C. P., Visco, S. J. & De Jonghe, L. C. Nanostructured solid oxide fuel cell electrodes.
_Nano Lett._ 7, 2136–2141 (2007). Article CAS Google Scholar * Kurokawa, H., Sholklapper, T. Z., Jacobson, C. P., De Jonghe, L. C. & Visco, S. J. Ceria nanocoating for sulfur tolerant
Ni-based anodes of solid oxide fuel cells. _Electrochem. Solid State_ 10, B135–B138 (2007). Article CAS Google Scholar * Gorte, R. J. & Vohs, J. M. Nanostructured anodes for solid
oxide fuel cells. _Curr. Opin. Colloid Interface_ 14, 236–244 (2009). Article CAS Google Scholar * Gross, M. D., Vohs, J. M. & Gorte, R. J. A strategy for achieving high performance
with SOFC ceramic anodes. _Electrochem. Solid State_ 10, B65–B69 (2007). Article CAS Google Scholar * Marina, O. A., Bagger, C., Primdahl, S. & Mogensen, M. A solid oxide fuel cell
with a gadolinia-doped ceria anode: Preparation and performance. _Solid State Ion._ 123, 199–208 (1999). Article CAS Google Scholar * Tsai, T. & Barnett, S. A. Increased solid-oxide
fuel cell power density using interfacial ceria layer. _Solid State Ion._ 98, 191–196 (1997). Article CAS Google Scholar * Tsai, T. & Barnett, S. A. Effect of mixed-conducting
interfacial layers on solid oxide fuel cell anode performance. _J. Electrochem. Soc._ 145, 1696–1701 (1998). Article CAS Google Scholar * Murray, E. P., Tsai, T. & Barnett, S. A. A
direct-methane fuel cell with a ceria-based anode. _Nature_ 400, 649–651 (1999). Article CAS Google Scholar * Park, S., Vohs, J. M. & Gorte, R. J. Direct oxidation of hydrocarbons in
solid-oxide fuel cell. _Nature_ 404, 265–267 (2000). Article CAS Google Scholar * Fu, Q., Weber, A. & Flytzani-Stephanopoulos, M. Nanostructured Au–CeO2 catalysts for low-temperature
water-gas shift. _Catal. Lett._ 77, 87–95 (2001). Article CAS Google Scholar * Park, J. B. et al. High catalytic activity of Au/CeO_x_/TiO2(110) controlled by the nature of the
mixed-metal oxide at the nanometer level. _Proc. Natl Acad. Sci. USA_ 163, 4975–4980 (2009). Article Google Scholar * Rodriguez, J. A. et al. Activity of CeO_x_ and TiO_x_ nanoparticles
grown on Au(111) in the water–gas-shift reaction. _Science_ 318, 1757–1760 (2007). Article CAS Google Scholar * Trovarelli, A. Catalytic properties of ceria and CeO2-containing materials.
_Catal. Rev. Sci. Eng._ 38, 439–520 (1996). Article CAS Google Scholar * Eguchi, K., Setoguchi, T., Inoue, T. & Arai, H. Electrical properties of ceria-based oxides and their
application to solid oxide fuel cells. _Solid State Ion._ 52, 165–172 (1992). Article CAS Google Scholar * Setoguchi, T., Okamoto, K., Eguchi, K. & Arai, H. Effects of anode material
and fuel on anodic reaction of solid oxide fuel cells. _J. Electrochem. Soc._ 139, 2875–2880 (1992). Article CAS Google Scholar * Ruiz-Trejo, E. & Maier, J. Electronic transport in
single crystals of Gd-doped ceria. _J. Electrochem. Soc._ 154, 583–587 (2007). Article Google Scholar * Tuller, H. L. & Nowick, A. S. Small polaron electron-transport in reduced CeO2
single-crystals. _J. Phys. Chem. Solids_ 38, 859–867 (1977). Article CAS Google Scholar * Lai, W. & Haile, S. M. Impedance spectroscopy as a tool for chemical and electrochemical
analysis of mixed conductors: A case study of ceria. _J. Am. Ceram. Soc._ 88, 2979–2997 (2005). Article CAS Google Scholar * Lu, C., Worrell, W. L., Vohs, J. M. & Gorte, R. J. A
comparison of Cu-ceria-SDC and Au-ceria-SDC composites for SOFC anodes. _J. Electrochem. Soc._ 150, A1357–A1359 (2003). Article CAS Google Scholar * Lai, W. _Impedance Spectroscopy as a
Tool for the Electrochemical Study of Mixed Conducting Ceria_. PhD thesis, California Inst. Technology, (2006). * Chueh, W. C., Lai, W. & Haile, S. M. Electrochemical behavior of ceria
with selected metal electrodes. _Solid State Ion._ 179, 1036–1041 (2008). Article CAS Google Scholar * Ciucci, F., Chueh, W. C., Goodwin, D. G. & Haile, S. M. Surface reaction and
transport in mixed conductors with electrochemically-active surfaces: A 2-D numerical study of ceria. _Phys. Chem. Chem. Phys._ 13, 2121–2135 (2011). Article CAS Google Scholar *
DeCaluwe, S. C. et al. _In situ_ characterization of ceria oxidation states in high-temperature electrochemical cells with ambient pressure XPS. _J. Phys. Chem. C_ 114, 19853–19861 (2010).
Article CAS Google Scholar * Nakamura, T. et al. Determination of the reaction zone in gadolinia-doped ceria anode for solid oxide fuel cell. _J. Electrochem. Soc._ 155, 1244–1250 (2008).
Article Google Scholar * Nakamura, T. et al. Electrochemical behaviors of mixed conducting oxide anodes for solid oxide fuel cells. _J. Electrochem. Soc._ 155, 563–569 (2008). Article
Google Scholar * Zhang, C. J. et al. Measuring fundamental properties in operating solid oxide electrochemical cells by using in situ X-ray photoelectron spectroscopy. _Nature Mater._ 9,
944–949 (2010). Article CAS Google Scholar * Chen, M., Kim, B. H., Xu, Q., Ahn, B. G. & Huang, D. P. Effect of Ni content on the microstructure and electrochemical properties of
Ni-SDC anodes for IT-SOFC. _Solid State Ion._ 181, 1119–1124 (2010). Article CAS Google Scholar * McIntosh, S., Vohs, J. M. & Gorte, R. J. Effect of precious-metal dopants on SOFC
anodes for direct utilization of hydrocarbons. _Electrochem. Solid State_ 6, A240–A243 (2003). Article CAS Google Scholar * Uchida, H., Suzuki, S. & Watanabe, M. High performance
electrode for medium-temperature solid oxide fuel cells: Mixed conducting ceria-based anode with highly dispersed Ni electrocatalysts. _Electrochem. Solid State_ 6, A174–A177 (2003). Article
CAS Google Scholar * Uchida, H., Osuga, T. & Watanabe, M. High-performance electrode for medium-temperature solid oxide fuel cells: Control of microstructure of ceria-based anodes
with highly dispersed ruthenium electrocatalysts. _J. Electrochem. Soc._ 146, 1677–1682 (1999). Article CAS Google Scholar * Bessler, W. G. et al. Model anodes and anode models for
understanding the mechanism of hydrogen oxidation in solid oxide fuel cells. _Phys. Chem. Chem. Phys._ 12, 13888–13903 (2010). CAS Google Scholar * Bieberle, A., Meier, L. P. &
Gauckler, L. J. The electrochemistry of Ni pattern anodes used as solid oxide fuel cell model electrodes. _J. Electrochem. Soc._ 148, A646–A656 (2001). Article CAS Google Scholar *
Mizusaki, J., Amano, K., Yamauchi, S. & Fueki, K. Electrode-reaction at Pt,O2(g)/stabilized zirconia interfaces. Part II. Electrochemical measurements & analysis. _Solid State Ion._
22, 323–330 (1987). Article CAS Google Scholar * Mizusaki, J. et al. Kinetic studies of the reaction at the nickel pattern electrode on YSZ in H2–H2O atmospheres. _Solid State Ion._ 70,
52–58 (1994). Article Google Scholar * Wilson, J. R. et al. Three-dimensional reconstruction of a solid-oxide fuel-cell anode. _Nature Mater._ 5, 541–544 (2006). Article CAS Google
Scholar * Brauer, G. & Gradinger, H. Uber heterotype Mischphasen bei Seltenerdoxyden. I. _Z. Anorg. Allg. Chem._ 276, 209–226 (1954). Article CAS Google Scholar * Godickemeier, M.
& Gauckler, L. J. Engineering of solid oxide fuel cells with ceria-based electrolytes. _J. Electrochem. Soc._ 145, 414–421 (1998). Article CAS Google Scholar * Mogensen, M., Sammes,
N. M. & Tompsett, G. A. Physical, chemical and electrochemical properties of pure an doped ceria. _Solid State Ion._ 129, 63–94 (2000). Article CAS Google Scholar * Chueh, W. C. &
Haile, S. M. Electrochemical studies of capacitance in cerium oxide thin films and its relationship to anionic and electronic defect densities. _Phys. Chem. Chem. Phys._ 11, 8144–8148
(2009). Article CAS Google Scholar * Kobayashi, T., Wang, S., Dokiya, M., Tagawa, H. & Hashimoto, T. Oxygen nonstoichiometry of Ce1−ySmyO2−0.5y−x (y=0.1, 0.2). _Solid State Ion._ 126,
349–357 (1999). Article CAS Google Scholar * Chueh, W. C. _Electrochemical and Thermochemical Behavior of_ _C_ _e_ _O_2−_x_. PhD thesis, California Inst. Technology (2011). * Huang,
Y-H., Dass, R. I., Xing, Z-L. & Goodenough, J. B. Double perovskite as anode materials for solid-oxide fuel cells. _Science_ 312, 254–257 (2006). Article CAS Google Scholar * Tao, S.
& Irvine, J. T. S. A redox-stable efficient anode for solid-oxide fuel cells. _Nature Mater._ 2, 320–323 (2003). Article CAS Google Scholar * Zha, S., Rauch, W. & Liu, M.
Ni–Ce0.9Gd0.1O1.95 anode for GDC electrolyte-based low-temperature SOFCs. _Solid State Ion._ 166, 241–250 (2004). Article CAS Google Scholar * Muecke, U. P. et al. Electrochemical
performance of nanocrystalline nickel/gadolinia-doped ceria thin film anodes for solid oxide fuel cells. _Solid State Ion._ 178, 1762–1768 (2008). Article CAS Google Scholar * Primdahl,
S. & Mogensen, M. Mixed conductor anodes: Ni as electrocatalyst for hydrogen conversion. _Solid State Ion._ 152–153, 597–608 (2002). Article Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported in part by the Stanford Global Climate & Energy Project and by the National Science Foundation under contract number DMR-0604004. Additional
support was provided by the NSF through the Caltech Center for the Science and Engineering of Materials, a Materials Research Science and Engineering Center (DMR-052056). W.C.C. was also
supported by an appointment to the Sandia National Laboratories Truman Fellowship in National Security Science and Engineering, sponsored by Sandia Corporation (a wholly owned subsidiary of
Lockheed Martin Corporation) as Operator of Sandia National Laboratories under its US Department of Energy Contract No. DE-AC04-94AL85000. The authors also acknowledge C. M. Garland and D.
A. Boyd of Caltech and M. W. Clift of Sandia for their assistance with analytical measurements, K. L. Gu of Caltech for sample fabrication, F. Ciucci of University of Heidelberg for
numerical simulations and D. G. Goodwin and E. C. Brown of Caltech and F. El Gabaly and A. H. McDaniel of Sandia for valuable discussions. The Evans Analytical Group carried out focused ion
beam imaging, and for that effort the authors are particularly grateful to H. Deng. AUTHOR INFORMATION Author notes * William C. Chueh Present address: Present address: Sandia National
Laboratories, Livermore, California 94551, USA, * William C. Chueh and Yong Hao: These authors contributed equally to this work AUTHORS AND AFFILIATIONS * Materials Science, California
Institute of Technology, Pasadena, California 91125, USA William C. Chueh, Yong Hao, WooChul Jung & Sossina M. Haile Authors * William C. Chueh View author publications You can also
search for this author inPubMed Google Scholar * Yong Hao View author publications You can also search for this author inPubMed Google Scholar * WooChul Jung View author publications You can
also search for this author inPubMed Google Scholar * Sossina M. Haile View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS W.C.C. designed the
experiment, fabricated samples and carried out analytical and electrochemical characterizations. Y.H. developed the fabrication methodology for dense electrochemical cells and carried out
the sample preparations and characterizations. W.J. fabricated and characterized porous electrochemical cells. S.M.H. guided and supervised the work. CORRESPONDING AUTHOR Correspondence to
Sossina M. Haile. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Information
(PDF 1606 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chueh, W., Hao, Y., Jung, W. _et al._ High electrochemical activity of the oxide phase in
model ceria–Pt and ceria–Ni composite anodes. _Nature Mater_ 11, 155–161 (2012). https://doi.org/10.1038/nmat3184 Download citation * Received: 01 April 2011 * Accepted: 26 October 2011 *
Published: 04 December 2011 * Issue Date: February 2012 * DOI: https://doi.org/10.1038/nmat3184 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
