New metastable form of ice and its role in the homogeneous crystallization of water
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

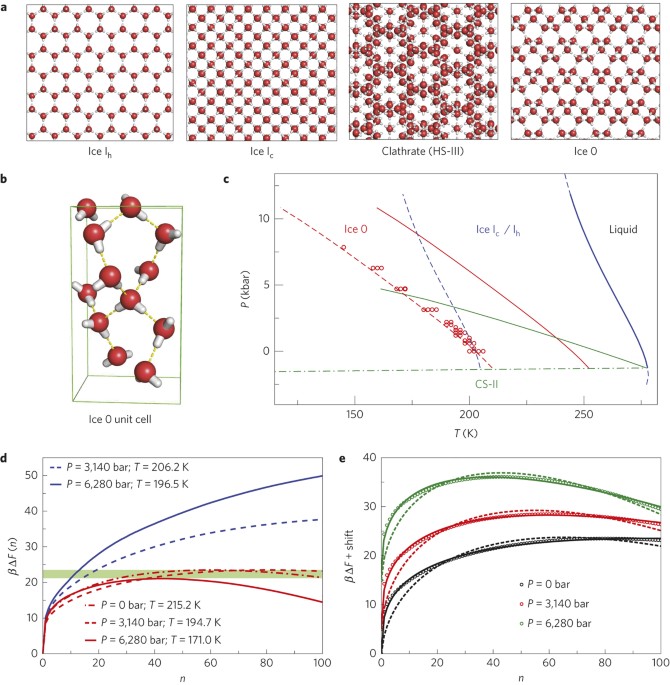
ABSTRACT The homogeneous crystallization of water at low temperature is believed to occur through the direct nucleation of cubic (Ic) and hexagonal (Ih) ices. Here, we provide evidence from
molecular simulations that the nucleation of ice proceeds through the formation of a new metastable phase, which we name Ice 0. We find that Ice 0 is structurally similar to the supercooled
liquid, and that on growth it gradually converts into a stacking of Ice Ic and Ih. We suggest that this mechanism provides a thermodynamic explanation for the location and pressure
dependence of the homogeneous nucleation temperature, and that Ice 0 controls the homogeneous nucleation of low-pressure ices, acting as a precursor to crystallization in accordance with
Ostwald’s step rule of phases. Our findings show that metastable crystalline phases of water may play roles that have been largely overlooked. Access through your institution Buy or
subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online
access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which
are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
TEMPERATURE-DEPENDENT KINETIC PATHWAYS OF HETEROGENEOUS ICE NUCLEATION COMPETING BETWEEN CLASSICAL AND NON-CLASSICAL NUCLEATION Article Open access 16 August 2021 METASTABLE WATER AT
SEVERAL COMPRESSION RATES AND ITS FREEZING KINETICS INTO ICE VII Article Open access 19 September 2024 MICROSCOPIC ORDERING OF SUPERCOOLED WATER ON THE ICE BASAL FACE Article Open access 19
May 2023 REFERENCES * Eisenberg, D. & Kauzmann, W. _The Structure and Properties of Water_ (Oxford Univ. Press, 1969). Google Scholar * Angell, C. A. Formation of glasses from liquids
and biopolymers. _Science_ 267, 1924–1935 (1995). Article CAS Google Scholar * Mishima, O. & Stanley, H. The relationship between liquid, supercooled and glassy water. _Nature_ 396,
329–335 (1998). Article CAS Google Scholar * Debenedetti, P. Supercooled and glassy water. _J. Phys. Condens. Matter_ 15, R1669–R1726 (2003). Article CAS Google Scholar * Pruppacher,
H. R. A new look at homogeneous ice nucleation in supercooled water drops. _J. Atmos. Sci._ 52, 1924–1933 (1995). Article Google Scholar * Rosenfeld, D. & Woodley, W. L. Deep
convective clouds with sustained supercooled liquid water down to −37.5 °C. _Nature_ 405, 440–442 (2000). Article CAS Google Scholar * Koop, T., Luo, B., Tsias, A. & Peter, T. Water
activity as the determinant for homogeneous ice nucleation in aqueous solutions. _Nature_ 406, 611–614 (2000). Article CAS Google Scholar * Soper, A. K. Structural transformations in
amorphous ice and supercooled water and their relevance to the phase diagram of water. _Mol. Phys._ 106, 2053–2076 (2008). Article CAS Google Scholar * Morishige, K. & Nobuoka, K.
X-ray diffraction studies of freezing and melting of water confined in a mesoporous adsorbent (MCM-41). _J. Chem. Phys._ 107, 6965–6969 (1997). Article CAS Google Scholar * Jelassi, J. et
al. Studies of water and ice in hydrophilic and hydrophobic mesoporous silicas: pore characterisation and phase transformations. _Phys. Chem. Chem. Phys._ 12, 2838–2849 (2010). Article CAS
Google Scholar * Hansen, T., Koza, M. & Kuhs, W. Formation and annealing of cubic ice: I. Modelling of stacking faults. _J. Phys. Condens. Matter_ 20, 285104 (2008). Article Google
Scholar * Shilling, J. et al. Measurements of the vapor pressure of cubic ice and their implications for atmospheric ice clouds. _Geophys. Res. Lett._ 33, L17801 (2006). Article Google
Scholar * Kobayashi, M. & Tanaka, H. Relationship between the phase diagram, the glass-forming ability, and the fragility of a water/salt mixture. _J. Phys. Chem. B_ 115, 14077–14090
(2011). Article CAS Google Scholar * Mayer, E. & Hallbrucker, A. Cubic ice from liquid water. _Nature_ 325, 601–602 (1987). Article CAS Google Scholar * Kohl, I., Mayer, E. &
Hallbrucker, A. The glassy water–cubic ice system: A comparative study by X-ray diffraction and differential scanning calorimetry. _Phys. Chem. Chem. Phys._ 2, 1579–1586 (2000). Article CAS
Google Scholar * Murray, B. J. & Bertram, A. K. Formation and stability of cubic ice in water droplets. _Phys. Chem. Chem. Phys._ 8, 186–192 (2006). Article CAS Google Scholar *
Malkin, T. L., Murray, B. J., Brukhno, A. V., Anwar, J. & Salzmann, C. G. Structure of ice crystallized from supercooled water. _Proc. Natl Acad. Sci. USA_ 109, 1041–1045 (2012). Article
CAS Google Scholar * Svishchev, I. M. & Kusalik, P. G. Crystallization of liquid water in a molecular dynamics simulation. _Phys. Rev. Lett._ 73, 975–978 (1994). Article CAS Google
Scholar * Matsumoto, M., Saito, S. & Ohmine, I. Molecular dynamics simulation of the ice nucleation and growth process leading to water freezing. _Nature_ 416, 409–413 (2002). Article
CAS Google Scholar * Moore, E. B. & Molinero, V. Is it cubic? Ice crystallization from deeply supercooled water. _Phys. Chem. Chem. Phys._ 13, 20008–20016 (2011). Article CAS
Google Scholar * Li, T., Donadio, D., Russo, G. & Galli, G. Homogeneous ice nucleation from supercooled water. _Phys. Chem. Chem. Phys._ 13, 19807–19813 (2011). Article CAS Google
Scholar * Reinhardt, A., Doye, J. P., Noya, E. G. & Vega, C. Local order parameters for use in driving homogeneous ice nucleation with all-atom models of water. _J. Chem. Phys._ 137,
194504–194504 (2012). Article Google Scholar * Moore, E. & Molinero, V. Structural transformation in supercooled water controls the crystallization rate of ice. _Nature_ 479, 506–508
(2011). Article CAS Google Scholar * Zhao, Z. et al. Tetragonal allotrope of group 14 elements. _J. Am. Chem. Soc._ 134, 12362–12365 (2012). Article CAS Google Scholar * Abascal, J.
& Vega, C. A general purpose model for the condensed phases of water: TIP4P/2005. _J. Chem. Phys._ 123, 234505 (2005). Article CAS Google Scholar * Sanz, E., Vega, C., Abascal, J.
& MacDowell, L. Phase diagram of water from computer simulation. _Phys. Rev. Lett._ 92, 255701 (2004). Article CAS Google Scholar * Jacobson, L. C., Hujo, W. & Molinero, V.
Thermodynamic stability and growth of guest-free clathrate hydrates: a low-density crystal phase of water. _J. Phys. Chem. B_ 113, 10298–10307 (2009). Article CAS Google Scholar * Romano,
F., Sanz, E. & Sciortino, F. Crystallization of tetrahedral patchy particles in silico. _J. Chem. Phys._ 134, 174502 (2011). Article Google Scholar * Ghiringhelli, L. M. et al.
State-of-the-art models for the phase diagram of carbon and diamond nucleation. _Mol. Phys._ 106, 2011–2038 (2008). Article CAS Google Scholar * Ten Wolde, P. R., Ruiz-Montero, M. J.
& Frenkel, D. Numerical evidence for bcc ordering at the surface of a critical fcc nucleus. _Phys. Rev. Lett._ 75, 2714–2717 (1995). Article CAS Google Scholar * Santra, M., Singh, R.
S. & Bagchi, B. Nucleation of a stable solid from melt in the presence of multiple metastable intermediate phases: Wetting, Ostwald step rule and vanishing polymorphs. _J. Phys. Chem.
B_ 117, 13154–13163 (2013). Article CAS Google Scholar * Lundrigan, S. E. & Saika-Voivod, I. Test of classical nucleation theory and mean first-passage time formalism on
crystallization in the Lennard-Jones liquid. _J. Chem. Phys._ 131, 104503 (2009). Article Google Scholar * Sanz, E. et al. Homogeneous ice nucleation at moderate supercooling from
molecular simulation. _J. Am. Chem. Soc._ 135, 15008–15017 (2013). Article CAS Google Scholar * Stillinger, F. H. Water revisited. _Science_ 209, 451–457 (1980). Article CAS Google
Scholar * Russo, J. & Tanaka, H. Understanding water’s anomalies with locally favored structures. _Nature Commun._ 5, 3556 (2014). Article Google Scholar * Ghiringhelli, L. M.,
Valeriani, C., Meijer, E. & Frenkel, D. Local structure of liquid carbon controls diamond nucleation. _Phys. Rev. Lett._ 99, 055702 (2007). Article CAS Google Scholar * Kawasaki, T.
& Tanaka, H. Formation of crystal nucleus from liquid. _Proc. Natl Acad. Sci. USA_ 107, 14036–14041 (2010). Article CAS Google Scholar * Russo, J. & Tanaka, H. The microscopic
pathway to crystallization in supercooled liquids. _Sci. Rep._ 2, 505 (2012). Article Google Scholar * Russo, J. & Tanaka, H. Selection mechanism of polymorphs in the crystal
nucleation of the Gaussian core model. _Soft Matter_ 8, 4206–4215 (2012). Article CAS Google Scholar * Tanaka, H. Bond orientational order in liquids: Towards a unified description of
water-like anomalies, liquid–liquid transition, glass transition, and crystallization. _Eur. Phys. J. E_ 35, 1–84 (2012). Article CAS Google Scholar * Seidl, M., Amann-Winkel, K., Handle,
P. H., Zifferer, G. & Loerting, T. From parallel to single crystallization kinetics in high-density amorphous ice. _Phys. Rev. B_ 88, 174105 (2013). Article Google Scholar * Marchand,
D. J., Hsiao, E. & Kim, S. H. Non-contact AFM imaging in water using electrically-driven cantilever vibration. _Langmuir_ 29, 6762–6769 (2013). Article CAS Google Scholar * Lied, A.,
Dosch, H. & Bilgram, J. H. Surface melting of ice I_h_ single crystals revealed by glancing angle X-ray scattering. _Phys. Rev. Lett._ 72, 3554–3557 (1994). Article CAS Google Scholar
* Vega, C., Sanz, E., Abascal, J. & Noya, E. Determination of phase diagrams via computer simulation: Methodology and applications to water, electrolytes and proteins. _J. Phys.
Condens. Matter_ 20, 153101 (2008). Article Google Scholar * Steinhardt, P. J., Nelson, D. R. & Ronchetti, M. Bond-orientational order in liquids and glasses. _Phys. Rev. B_ 28,
784–805 (1983). Article CAS Google Scholar * Lechner, W. & Dellago, C. Accurate determination of crystal structures based on averaged local bond order parameters. _J. Chem. Phys._
129, 114707 (2008). Article Google Scholar * Auer, S. & Frenkel, D. Prediction of absolute crystal-nucleation rate in hard-sphere colloids. _Nature_ 409, 1020–1023 (2001). Article CAS
Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to F. Sciortino for a critical reading of the manuscript and to J. Doye and A. Reinhardt for useful discussions. This
study was partly supported by Grants-in-Aid for Scientific Research (S) and Specially Promoted Research from the Japan Society for the Promotion of Science (JSPS), the Aihara Project, the
FIRST programme from JSPS, initiated by the Council for Science and Technology Policy (CSTP), a JSPS short-term fellowship for F.R., and a JSPS Postdoctoral Fellowship for J.R. AUTHOR
INFORMATION Author notes * John Russo and Flavio Romano: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Institute of Industrial Science, University of Tokyo,
4-6-1 Komaba, Meguro-ku Tokyo 153-8505, Japan, John Russo, Flavio Romano & Hajime Tanaka * Department of Chemistry, Physical and Theoretical Chemistry Laboratory, University of Oxford,
South Parks Road Oxford OX1 3QZ, UK, Flavio Romano Authors * John Russo View author publications You can also search for this author inPubMed Google Scholar * Flavio Romano View author
publications You can also search for this author inPubMed Google Scholar * Hajime Tanaka View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
J.R. and F.R. performed the numerical simulations and the data analysis. H.T. proposed and supervised the study. All authors discussed the results and contributed to the writing of the
manuscript. CORRESPONDING AUTHOR Correspondence to Hajime Tanaka. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION Supplementary Information (PDF 2172 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Russo, J., Romano, F. & Tanaka, H.
New metastable form of ice and its role in the homogeneous crystallization of water. _Nature Mater_ 13, 733–739 (2014). https://doi.org/10.1038/nmat3977 Download citation * Received: 22
September 2013 * Accepted: 08 April 2014 * Published: 18 May 2014 * Issue Date: July 2014 * DOI: https://doi.org/10.1038/nmat3977 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
