Direct detection of dna methylation during single-molecule, real-time sequencing
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

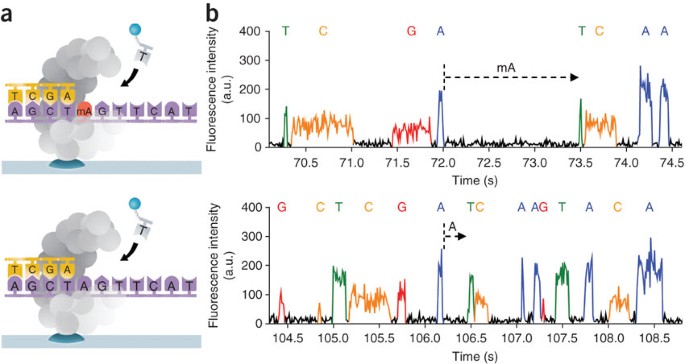
ABSTRACT We describe the direct detection of DNA methylation, without bisulfite conversion, through single-molecule, real-time (SMRT) sequencing. In SMRT sequencing, DNA polymerases catalyze
the incorporation of fluorescently labeled nucleotides into complementary nucleic acid strands. The arrival times and durations of the resulting fluorescence pulses yield information about
polymerase kinetics and allow direct detection of modified nucleotides in the DNA template, including N6-methyladenine, 5-methylcytosine and 5-hydroxymethylcytosine. Measurement of
polymerase kinetics is an intrinsic part of SMRT sequencing and does not adversely affect determination of primary DNA sequence. The various modifications affect polymerase kinetics
differently, allowing discrimination between them. We used these kinetic signatures to identify adenine methylation in genomic samples and found that, in combination with circular consensus
sequencing, they can enable single-molecule identification of epigenetic modifications with base-pair resolution. This method is amenable to long read lengths and will likely enable mapping
of methylation patterns in even highly repetitive genomic regions. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution
ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article *
Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn
about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS DIRECT ENZYMATIC SEQUENCING OF 5-METHYLCYTOSINE AT SINGLE-BASE RESOLUTION
Article 15 June 2023 DIRECT TRANSPOSITION OF NATIVE DNA FOR SENSITIVE MULTIMODAL SINGLE-MOLECULE SEQUENCING Article Open access 09 May 2024 SEQUENCE TERMINUS DEPENDENT PCR FOR SITE-SPECIFIC
MUTATION AND MODIFICATION DETECTION Article Open access 01 March 2023 REFERENCES * Marinus, M.G. & Casadesus, J. Roles of DNA adenine methylation in host-pathogen interactions: mismatch
repair, transcriptional regulation, and more. _FEMS Microbiol. Rev._ 33, 488–503 (2009). Article CAS Google Scholar * Cokus, S.J. et al. Shotgun bisulphite sequencing of the _Arabidopsis_
genome reveals DNA methylation patterning. _Nature_ 452, 215–219 (2008). Article CAS Google Scholar * Lister, R. et al. Highly integrated single-base resolution maps of the epigenome in
_Arabidopsis_. _Cell_ 133, 523–536 (2008). Article CAS Google Scholar * Gardiner-Garden, M. & Frommer, M. CpG islands in vertebrate genomes. _J. Mol. Biol._ 196, 261–282 (1987).
Article CAS Google Scholar * Saxonov, S., Berg, P. & Brutlag, D.L. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters.
_Proc. Natl. Acad. Sci. USA_ 103, 1412–1417 (2006). Article CAS Google Scholar * Pomraning, K.R., Smith, K.M. & Freitag, M. Genome-wide high throughput analysis of DNA methylation in
eukaryotes. _Methods_ 47, 142–150 (2009). Article CAS Google Scholar * Jaenisch, R. & Bird, A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and
environmental signals. _Nat. Genet._ 33 (Suppl.), 245–254 (2003). Article CAS Google Scholar * Holliday, R. & Pugh, J.E. DNA modification mechanisms and gene activity during
development. _Science_ 187, 226–232 (1975). Article CAS Google Scholar * Riggs, A.D. X inactivation, differentiation, and DNA methylation. _Cytogenet. Cell Genet._ 14, 9–25 (1975).
Article CAS Google Scholar * Li, E., Bestor, T.H. & Jaenisch, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. _Cell_ 69, 915–926 (1992). Article
CAS Google Scholar * Razin, A. & Shemer, R. DNA methylation in early development. _Hum. Mol. Genet._ 4 Spec No, 1751–1755 (1995). Article CAS Google Scholar * Jones, P.A. &
Baylin, S.B. The fundamental role of epigenetic events in cancer. _Nat. Rev. Genet._ 3, 415–428 (2002). Article CAS Google Scholar * Jones, P.A. & Laird, P.W. Cancer epigenetics comes
of age. _Nat. Genet._ 21, 163–167 (1999). Article CAS Google Scholar * Robertson, K.D. DNA methylation and human disease. _Nat. Rev. Genet._ 6, 597–610 (2005). Article CAS Google
Scholar * Lister, R. et al. Human DNA methylomes at base resolution show widespread epigenomic differences. _Nature_ 462, 315–322 (2009). Article CAS Google Scholar * Kriaucionis, S.
& Heintz, N. The nuclear DNA base 5-hydroxymethylcytosine is present in purkinje neurons and the brain. _Science_ 324, 929–930 (2009). Article CAS Google Scholar * Tahiliani, M. et
al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. _Science_ 324, 930–935 (2009). Article CAS Google Scholar * Lister, R. & Ecker,
J.R. Finding the fifth base: genome-wide sequencing of cytosine methylation. _Genome Res._ 19, 959–966 (2009). Article CAS Google Scholar * Meissner, A. et al. Genome-scale DNA
methylation maps of pluripotent and differentiated cells. _Nature_ 454, 766–770 (2008). Article CAS Google Scholar * Clark, S.J., Statham, A., Stirzaker, C., Molloy, P.L. & Frommer,
M. DNA methylation: bisulphite modification and analysis. _Nat. Protocols_ 1, 2353–2364 (2006). Article CAS Google Scholar * Hayatsu, H. & Shiragami, M. Reaction of bisulfite with the
5-hydroxymethyl group in pyrimidines and in phage DNAs. _Biochemistry_ 18, 632–637 (1979). Article CAS Google Scholar * Huang, Y. et al. The behaviour of 5-hydroxymethylcytosine in
bisulfite sequencing. _PLoS One_ 5, e8888 (2010). Article Google Scholar * Tardy-Planechaud, S., Fujimoto, J., Lin, S.S. & Sowers, L.C. Solid phase synthesis and restriction
endonuclease cleavage of oligodeoxynucleotides containing 5-(hydroxymethyl)-cytosine. _Nucleic Acids Res._ 25, 553–559 (1997). Article CAS Google Scholar * Clarke, J. et al. Continuous
base identification for single-molecule nanopore DNA sequencing. _Nat. Nanotechnol._ 4, 265–270 (2009). Article CAS Google Scholar * Eid, J. et al. Real-time DNA sequencing from single
polymerase molecules. _Science_ 323, 133–138 (2009). Article CAS Google Scholar * Levene, M.J. et al. Zero-mode waveguides for single-molecule analysis at high concentrations. _Science_
299, 682–686 (2003). Article CAS Google Scholar * Wong, I., Patel, S.S. & Johnson, K.A. An induced-fit kinetic mechanism for DNA replication fidelity: direct measurement by
single-turnover kinetics. _Biochemistry_ 30, 526–537 (1991). Article CAS Google Scholar * Hsu, G.W., Ober, M., Carell, T. & Beese, L.S. Error-prone replication of oxidatively damaged
DNA by a high-fidelity DNA polymerase. _Nature_ 431, 217–221 (2004). Article CAS Google Scholar * Berman, A.J. et al. Structures of phi29 DNA polymerase complexed with substrate: the
mechanism of translocation in B-family polymerases. _EMBO J._ 26, 3494–3505 (2007). Article CAS Google Scholar * Lundquist, P.M. et al. Parallel confocal detection of single molecules in
real time. _Opt. Lett._ 33, 1026–1028 (2008). Article Google Scholar * Foquet, M. et al. Improved fabrication of zero-mode waveguides for single-molecule detection. _J. Appl. Phys._ 103,
034301 (2008). Article Google Scholar * Korlach, J. et al. Selective aluminum passivation for targeted immobilization of single DNA polymerase molecules in zero-mode waveguide
nanostructures. _Proc. Natl. Acad. Sci. USA_ 105, 1176–1181 (2008). Article CAS Google Scholar * Korlach, J. et al. Long, processive enzymatic DNA synthesis using 100% dye-labeled
terminal phosphate-linked nucleotides. _Nucleosides Nucleotides Nucleic Acids_ 27, 1072–1082 (2008). Article CAS Google Scholar * Jolliffe, I.T. _Principal Component Analysis_ 2nd edn.
(Springer-Verlag, New York, 2002). Download references ACKNOWLEDGEMENTS We thank the entire staff at Pacific Biosciences, in particular J. Londry and D. Kolesnikov for sample preparation; E.
Mollova, M. Berhe and J. Yen for running sequencing experiments; J. Sorenson, J. Chin, A. Kislyuk and D. Holden for help with data analysis; and E. Schadt and J. Eid for helpful
discussions. This work was supported by US National Human Genome Research Institute grant 1RC2HG005618-01. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Pacific Biosciences, Menlo Park,
California, USA Benjamin A Flusberg, Dale R Webster, Jessica H Lee, Kevin J Travers, Eric C Olivares, Tyson A Clark, Jonas Korlach & Stephen W Turner Authors * Benjamin A Flusberg View
author publications You can also search for this author inPubMed Google Scholar * Dale R Webster View author publications You can also search for this author inPubMed Google Scholar *
Jessica H Lee View author publications You can also search for this author inPubMed Google Scholar * Kevin J Travers View author publications You can also search for this author inPubMed
Google Scholar * Eric C Olivares View author publications You can also search for this author inPubMed Google Scholar * Tyson A Clark View author publications You can also search for this
author inPubMed Google Scholar * Jonas Korlach View author publications You can also search for this author inPubMed Google Scholar * Stephen W Turner View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS B.A.F., K.J.T., J.K., J.H.L. and S.W.T. designed the experiments. E.C.O. and T.A.C. prepared fosmid library constructs. B.A.F.
conducted the sequencing experiments. D.R.W. and B.A.F. analyzed data. B.A.F., J.K., S.W.T., E.C.O., D.R.W. and T.A.C. wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Stephen W
Turner. ETHICS DECLARATIONS COMPETING INTERESTS All of the authors are employees of Pacific Biosciences. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES Supplementary Figures 1–6,
Supplementary Table 1 and Supplementary Note 1 (PDF 511 kb) SUPPLEMENTARY DATA IPD values at fosmid GATC positions (XLS 58 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Flusberg, B., Webster, D., Lee, J. _et al._ Direct detection of DNA methylation during single-molecule, real-time sequencing. _Nat Methods_ 7, 461–465 (2010).
https://doi.org/10.1038/nmeth.1459 Download citation * Received: 31 December 2009 * Accepted: 08 April 2010 * Published: 09 May 2010 * Issue Date: June 2010 * DOI:
https://doi.org/10.1038/nmeth.1459 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
