Magnetic nanoparticle–mediated massively parallel mechanical modulation of single-cell behavior
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

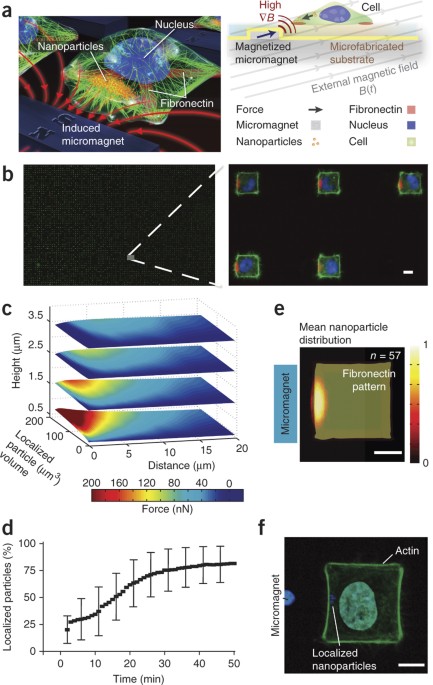
We report a technique for generating controllable, time-varying and localizable forces on arrays of cells in a massively parallel fashion. To achieve this, we grow magnetic
nanoparticle–dosed cells in defined patterns on micromagnetic substrates. By manipulating and coalescing nanoparticles within cells, we apply localized nanoparticle-mediated forces
approaching cellular yield tensions on the cortex of HeLa cells. We observed highly coordinated responses in cellular behavior, including the p21-activated kinase–dependent generation of
active, leading edge–type filopodia and biasing of the metaphase plate during mitosis. The large sample size and rapid sample generation inherent to this approach allow the analysis of cells
at an unprecedented rate: in a single experiment, potentially tens of thousands of cells can be stimulated for high statistical accuracy in measurements. This technique shows promise as a
tool for both cell analysis and control.
This work was partially supported through the US National Institutes of Health Director's New Innovator Award (1DP2OD007113). The authors thank M. Bachman and N. Gunn (University of
California, Irvine) for samples of PSR; J. Harrison, M. Glickman and I. Goldberg for assistance with the permalloy electroplating bath; members of the UCLA Advanced Light Microscopy
Spectroscopy facility for assistance with confocal microscopy; K. Lin for high-speed imaging assistance; I. Williams for running FACS; and engineers of the UCLA Nanolab for processing
assistance.
Department of Bioengineering, University of California, Los Angeles (UCLA), Los Angeles, California, USA
Department of Electrical Engineering, UCLA, Los Angeles, California, USA
California NanoSystems Institute, UCLA, Los Angeles, California, USA
Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, California, USA
P.T. and D.D.C. contributed to the initial concept. P.T. and J.W.J. contributed to the fabrication design. D.D.C. and P.T. designed the integration of magnetic elements and single cells.
P.T. developed the final fabrication and cell-patterning protocols. P.T. fabricated the micromagnetic slides and conducted the cell experiments. J.W.J. and P.T. discussed the finite-element
simulation. P.T. designed the numerical analysis flow. P.T. and D.D.C. discussed and analyzed the numerical results. All authors wrote the manuscript.
Nanoparticle assembly occurs over a period of ~30 min at the left edge of the cell. As nanoparticles begin coalescing at the membrane edge, small clusters of nanoparticles enter temporary
filopodial protrusions that extend beyond the edge. This effect continues until around the 1-h mark, when the cell membrane begins to yield under the high tension until finally the
nanoparticle cluster exhibits a 'pull-in' instability, and the entire nanoparticle cluster protrudes from the edge of the cell membrane. Cell cytoplasm is labeled with calcein AM. (MOV 1472
kb)
Video displays the trajectories of two magnetic beads moving along the substrate surface towards the magnetized elements using resins with thicknesses of 2.5 and 5.3 μm, respectively. (AVI
970 kb)
At the z planes where the cell membrane is under magnetic nanoparticle–induced tension, local effects are observed including (i) local deformation of the flanking stress fiber caused by the
applied mechanical tension and (ii) flanking actin-rich protrusions emanating from the regions of highest mechanical deformation. Positive myosin-X staining at the tips of protrusions
indicate induced active, ECM-attachable filopodia. (AVI 479 kb)
This colocalization occurs whether or not the particular cell is expressing a high filopodial asymmetry and is distinct at regions directly above where the nanoparticles are localized. (AVI
1042 kb)
The time-lapse video shows a single cell adhering to an I-shaped fibronectin pattern as it divides under high nanoparticle-induced tension. As the cell undergoes and completes mitosis, the
cell divides biased in the direction of the force generated by the magnetic nanoparticles. Upon successful division, both cells adhere and move normally. The nanoparticles remain only in one
of the daughter cells. (MOV 936 kb)
Anyone you share the following link with will be able to read this content:
