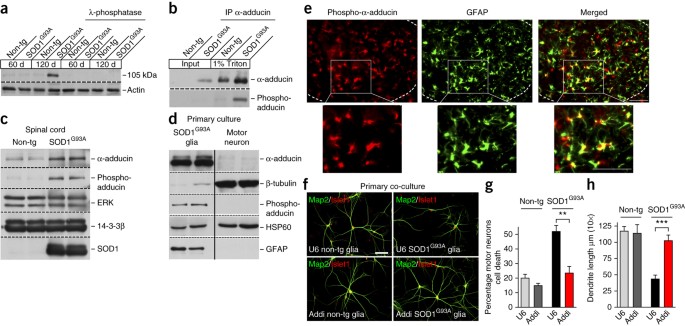
An α2-na/k atpase/α-adducin complex in astrocytes triggers non–cell autonomous neurodegeneration
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Perturbations of astrocytes trigger neurodegeneration in several diseases, but the glial cell–intrinsic mechanisms that induce neurodegeneration remain poorly understood. We found
that a protein complex of α2-Na/K ATPase and α-adducin was enriched in astrocytes expressing mutant superoxide dismutase 1 (SOD1), which causes familial amyotrophic lateral sclerosis (ALS).
Knockdown of α2-Na/K ATPase or α-adducin in mutant SOD1 astrocytes protected motor neurons from degeneration, including in mutant SOD1 mice _in vivo_. Heterozygous disruption of the α2-Na/K
ATPase gene suppressed degeneration _in vivo_ and increased the lifespan of mutant SOD1 mice. The pharmacological agent digoxin, which inhibits Na/K ATPase activity, protected motor neurons
from mutant SOD1 astrocyte–induced degeneration. Notably, α2-Na/K ATPase and α-adducin were upregulated in spinal cord of sporadic and familial ALS patients. Collectively, our findings
define chronic activation of the α2-Na/K ATPase/α-adducin complex as a critical glial cell–intrinsic mechanism of non–cell autonomous neurodegeneration, with implications for potential
therapies for neurodegenerative diseases. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access
through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS OLIGODENDROCYTE-ASTROCYTE CROSSTALK IN PARKINSON’S DISEASE MEDIATES NEURONAL FERROPTOSIS VIA
THE FGF SIGNALING PATHWAY Article Open access 23 May 2025 PROTEOMIC ANALYSIS LINKS ALTERATIONS OF BIOENERGETICS, MITOCHONDRIA-ER INTERACTIONS AND PROTEOSTASIS IN HIPPOCAMPAL ASTROCYTES FROM
3XTG-AD MICE Article Open access 18 August 2020 FERROPTOSIS MEDIATES SELECTIVE MOTOR NEURON DEATH IN AMYOTROPHIC LATERAL SCLEROSIS Article 02 December 2021 REFERENCES * Allen, N.J. &
Barres, B.A. Signaling between glia and neurons: focus on synaptic plasticity. _Curr. Opin. Neurobiol._ 15, 542–548 (2005). Article CAS PubMed Google Scholar * Fields, R.D. &
Stevens-Graham, B. New insights into neuron-glia communication. _Science_ 298, 556–562 (2002). Article CAS PubMed PubMed Central Google Scholar * Ilieva, H., Polymenidou, M. &
Cleveland, D.W. Non–cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. _J. Cell Biol._ 187, 761–772 (2009). Article CAS PubMed PubMed Central Google Scholar *
McGann, J.C., Lioy, D.T. & Mandel, G. Astrocytes conspire with neurons during progression of neurological disease. _Curr. Opin. Neurobiol._ 22, 850–858 (2012). Article CAS PubMed
PubMed Central Google Scholar * Garden, G.A. et al. Polyglutamine-expanded ataxin-7 promotes non–cell-autonomous purkinje cell degeneration and displays proteolytic cleavage in ataxic
transgenic mice. _J. Neurosci._ 22, 4897–4905 (2002). Article CAS PubMed PubMed Central Google Scholar * Yoo, S.Y. et al. SCA7 knockin mice model human SCA7 and reveal gradual
accumulation of mutant ataxin-7 in neurons and abnormalities in short-term plasticity. _Neuron_ 37, 383–401 (2003). Article CAS PubMed Google Scholar * Evert, B.O. et al. Inflammatory
genes are upregulated in expanded ataxin-3–expressing cell lines and spinocerebellar ataxia type 3 brains. _J. Neurosci._ 21, 5389–5396 (2001). Article CAS PubMed PubMed Central Google
Scholar * Clement, A.M. et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. _Science_ 302, 113–117 (2003). Article CAS PubMed Google Scholar *
Yamanaka, K. et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. _Nat. Neurosci._ 11, 251–253 (2008). Article CAS PubMed PubMed Central
Google Scholar * Faideau, M. et al. _In vivo_ expression of polyglutamine-expanded huntingtin by mouse striatal astrocytes impairs glutamate transport: a correlation with Huntington's
disease subjects. _Hum. Mol. Genet._ 19, 3053–3067 (2010). Article CAS PubMed PubMed Central Google Scholar * Shin, J.Y. et al. Expression of mutant huntingtin in glial cells
contributes to neuronal excitotoxicity. _J. Cell Biol._ 171, 1001–1012 (2005). Article CAS PubMed PubMed Central Google Scholar * Rothstein, J.D., Van Kammen, M., Levey, A.I., Martin,
L.J. & Kuncl, R.W. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. _Ann. Neurol._ 38, 73–84 (1995). Article CAS PubMed Google Scholar * Boillée,
S. et al. Onset and progression in inherited ALS determined by motor neurons and microglia. _Science_ 312, 1389–1392 (2006). Article CAS PubMed Google Scholar * Nagai, M. et al.
Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. _Nat. Neurosci._ 10, 615–622 (2007). Article CAS PubMed PubMed Central Google Scholar *
Di Giorgio, F.P., Carrasco, M.A., Siao, M.C., Maniatis, T. & Eggan, K. Non–cell autonomous effect of glia on motor neurons in an embryonic stem cell–based ALS model. _Nat. Neurosci._
10, 608–614 (2007). Article CAS PubMed PubMed Central Google Scholar * Dion, P.A., Daoud, H. & Rouleau, G.A. Genetics of motor neuron disorders: new insights into pathogenic
mechanisms. _Nat. Rev. Genet._ 10, 769–782 (2009). Article CAS PubMed Google Scholar * Rosen, D.R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial
amyotrophic lateral sclerosis. _Nature_ 362, 59–62 (1993). Article CAS PubMed Google Scholar * Gurney, M.E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide
dismutase mutation. _Science_ 264, 1772–1775 (1994). Article CAS PubMed Google Scholar * Bruijn, L.I., Miller, T.M. & Cleveland, D.W. Unraveling the mechanisms involved in motor
neuron degeneration in ALS. _Annu. Rev. Neurosci._ 27, 723–749 (2004). Article CAS PubMed Google Scholar * Haidet-Phillips, A.M. et al. Astrocytes from familial and sporadic ALS patients
are toxic to motor neurons. _Nat. Biotechnol._ 29, 824–828 (2011). Article CAS PubMed PubMed Central Google Scholar * Goodman, L.S., Brunton, L.L., Chabner, B. & Knollmann, B.C.
_Goodman & Gilman's Pharmacological Basis of Therapeutics_ (McGraw-Hill, New York, 2011). * Lehtinen, M.K. et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress
responses and extends life span. _Cell_ 125, 987–1001 (2006). Article CAS PubMed Google Scholar * Matsuoka, Y., Li, X. & Bennett, V. Adducin: structure, function and regulation.
_Cell. Mol. Life Sci._ 57, 884–895 (2000). Article CAS PubMed Google Scholar * Robledo, R.F. et al. Targeted deletion of alpha-adducin results in absent beta- and gamma-adducin,
compensated hemolytic anemia, and lethal hydrocephalus in mice. _Blood_ 112, 4298–4307 (2008). Article CAS PubMed PubMed Central Google Scholar * Shan, X., Hu, J.H., Cayabyab, F.S.
& Krieger, C. Increased phospho-adducin immunoreactivity in a murine model of amyotrophic lateral sclerosis. _Neuroscience_ 134, 833–846 (2005). Article CAS PubMed Google Scholar *
Hu, J.H., Zhang, H., Wagey, R., Krieger, C. & Pelech, S.L. Protein kinase and protein phosphatase expression in amyotrophic lateral sclerosis spinal cord. _J. Neurochem._ 85, 432–442
(2003). Article CAS PubMed Google Scholar * Kaplan, J.H. Biochemistry of Na,K-ATPase. _Annu. Rev. Biochem._ 71, 511–535 (2002). Article CAS PubMed Google Scholar * Watts, A.G.,
Sanchez-Watts, G., Emanuel, J.R. & Levenson, R. Cell-specific expression of mRNAs encoding Na+, K(+)-ATPase alpha- and beta-subunit isoforms within the rat central nervous system. _Proc.
Natl. Acad. Sci. USA_ 88, 7425–7429 (1991). Article CAS PubMed PubMed Central Google Scholar * Moseley, A.E. et al. Deficiency in Na,K-ATPase alpha isoform genes alters spatial
learning, motor activity, and anxiety in mice. _J. Neurosci._ 27, 616–626 (2007). Article CAS PubMed PubMed Central Google Scholar * Huang, G. et al. Death receptor 6 (DR6) antagonist
antibody is neuroprotective in the mouse SOD1G93A model of amyotrophic lateral sclerosis. _Cell Death Dis._ 4, e841 (2013). Article CAS PubMed PubMed Central Google Scholar * Hartford,
A.K., Messer, M.L., Moseley, A.E., Lingrel, J.B. & Delamere, N.A. Na,K-ATPase alpha 2 inhibition alters calcium responses in optic nerve astrocytes. _Glia_ 45, 229–237 (2004). Article
PubMed Google Scholar * Nakahira, K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. _Nat.
Immunol._ 12, 222–230 (2011). Article CAS PubMed Google Scholar * Bulua, A.C. et al. Mitochondrial reactive oxygen species promote production of pro-inflammatory cytokines and are
elevated in TNFR1-associated periodic syndrome (TRAPS). _J. Exp. Med._ 208, 519–533 (2011). Article CAS PubMed PubMed Central Google Scholar * Zhou, R., Yazdi, A.S., Menu, P. &
Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. _Nature_ 469, 221–225 (2011). Article CAS PubMed Google Scholar * Wu, D.C., Re, D.B., Nagai, M., Ischiropoulos, H.
& Przedborski, S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. _Proc. Natl. Acad. Sci. USA_ 103, 12132–12137 (2006).
Article CAS PubMed PubMed Central Google Scholar * Phatnani, H.P. et al. Intricate interplay between astrocytes and motor neurons in ALS. _Proc. Natl. Acad. Sci. USA_ 110, E756–E765
(2013). Article PubMed PubMed Central Google Scholar * Brooks, B.R., Miller, R.G., Swash, M. & Munsat, T.L. El Escorial revisited: revised criteria for the diagnosis of amyotrophic
lateral sclerosis. _Amyotroph. Lateral Scler. Other Motor Neuron Disord._ 1, 293–299 (2000). Article CAS PubMed Google Scholar * Ellis, D.Z., Rabe, J. & Sweadner, K.J. Global loss of
Na,K-ATPase and its nitric oxide–mediated regulation in a transgenic mouse model of amyotrophic lateral sclerosis. _J. Neurosci._ 23, 43–51 (2003). Article CAS PubMed PubMed Central
Google Scholar * Martin, L.J. et al. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell
death. _J. Comp. Neurol._ 500, 20–46 (2007). Article CAS PubMed Google Scholar * Kaphzan, H., Buffington, S.A., Jung, J.I., Rasband, M.N. & Klann, E. Alterations in intrinsic
membrane properties and the axon initial segment in a mouse model of Angelman syndrome. _J. Neurosci._ 31, 17637–17648 (2011). Article CAS PubMed PubMed Central Google Scholar *
Kaphzan, H. et al. Genetic reduction of the alpha1 subunit of Na/K-ATPase corrects multiple hippocampal phenotypes in Angelman syndrome. _Cell Reports_ 4, 405–412 (2013). Article CAS
PubMed Google Scholar * Efendiev, R. et al. Hypertension-linked mutation in the adducin alpha-subunit leads to higher AP2-mu2 phosphorylation and impaired Na+,K+-ATPase trafficking in
response to GPCR signals and intracellular sodium. _Circ. Res._ 95, 1100–1108 (2004). Article CAS PubMed Google Scholar * Torielli, L. et al. alpha-adducin mutations increase Na/K pump
activity in renal cells by affecting constitutive endocytosis: implications for tubular Na reabsorption. _Am. J. Physiol. Renal Physiol._ 295, F478–F487 (2008). Article CAS PubMed Google
Scholar * Cusi, D. et al. Polymorphisms of alpha-adducin and salt sensitivity in patients with essential hypertension. _Lancet_ 349, 1353–1357 (1997). Article CAS PubMed Google Scholar
* Kennedy, D.J. et al. CD36 and Na/K-ATPase-alpha1 form a pro-inflammatory signaling loop in kidney. _Hypertension_ 61, 216–224 (2013). Article CAS PubMed Google Scholar * Liu, J.,
Kennedy, D.J., Yan, Y. & Shapiro, J.I. Reactive oxygen species modulation of Na/K-ATPase regulates fibrosis and renal proximal tubular sodium handling. _Int. J. Nephrol._ 2012, 381320
(2012). Article CAS PubMed PubMed Central Google Scholar * Wang, J.K. et al. Cardiac glycosides provide neuroprotection against ischemic stroke: discovery by a brain slice-based
compound screening platform. _Proc. Natl. Acad. Sci. USA_ 103, 10461–10466 (2006). Article CAS PubMed PubMed Central Google Scholar * Piccioni, F., Roman, B.R., Fischbeck, K.H. &
Taylor, J.P. A screen for drugs that protect against the cytotoxicity of polyglutamine-expanded androgen receptor. _Hum. Mol. Genet._ 13, 437–446 (2004). Article CAS PubMed Google Scholar
* Corcoran, L.J., Mitchison, T.J. & Liu, Q. A novel action of histone deacetylase inhibitors in a protein aggresome disease model. _Curr. Biol._ 14, 488–492 (2004). Article CAS
PubMed Google Scholar * Burkhardt, M.F. et al. A cellular model for sporadic ALS using patient-derived induced pluripotent stem cells. _Mol. Cell. Neurosci._ 56, 355–364 (2013). Article
CAS PubMed PubMed Central Google Scholar * Chandra, S., Gallardo, G., Fernandez-Chacon, R., Schluter, O.M. & Sudhof, T.C. Alpha-synuclein cooperates with CSPalpha in preventing
neurodegeneration. _Cell_ 123, 383–396 (2005). Article CAS PubMed Google Scholar * Gaudilliere, B., Shi, Y. & Bonni, A. RNA interference reveals a requirement for myocyte enhancer
factor 2A in activity-dependent neuronal survival. _J. Biol. Chem._ 277, 46442–46446 (2002). Article CAS PubMed Google Scholar * Gingras, M., Gagnon, V., Minotti, S., Durham, H.D. &
Berthod, F. Optimized protocols for isolation of primary motor neurons, astrocytes and microglia from embryonic mouse spinal cord. _J. Neurosci. Methods_ 163, 111–118 (2007). Article CAS
PubMed Google Scholar * Raoul, C. et al. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. _Nat. Med._ 11,
423–428 (2005). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank members of the Bonni laboratory for helpful discussions. We thank L. Zinman (University of
Toronto) for providing human patient tissue samples. This work was supported by a grant from the Edward R. and Anne G. Lefler Foundation (A.B.) and The Ruth L. Kirschstein National Research
Service Awards T32 5T32AG00222 (G.G.). Human spinal cord material provided from Northwestern University autopsy program is partially funded from the Les Turner ALS Foundation. Additional
human tissue samples were obtained from the Human Brain and Spinal Fluid Resource Center, which is sponsored by the National Institute of Neurological Disorders and Stroke and the US
National Institutes of Health, National Multiple Sclerosis Society, and Department of Veterans Affairs. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Neurobiology, Harvard
Medical School, Boston, Massachusetts, USA Gilbert Gallardo, Jessica Barowski & Azad Bonni * Department of Anatomy and Neurobiology, Washington University School of Medicine, St. Louis,
Missouri, USA Gilbert Gallardo & Azad Bonni * Department of Neurosciences, University of California, San Diego, La Jolla, California, USA., John Ravits * Department of Neurology,
Northwestern Feinberg School of Medicine, Chicago, Illinois, USA Teepu Siddique * Department of Molecular and Cellular Biology, Northwestern Feinberg School of Medicine, Chicago, Illinois,
USA Teepu Siddique * Department of Molecular Genetics, Biochemistry and Microbiology, College of Medicine, University of Cincinnati, Cincinnati, Ohio, USA Jerry B Lingrel * Tanz Centre for
Research in Neurodegenerative Diseases and Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada Janice Robertson * Department of Pathology,
Boston Children's Hospital and Harvard Medical School, Boston, Massachusetts, USA Hanno Steen Authors * Gilbert Gallardo View author publications You can also search for this author
inPubMed Google Scholar * Jessica Barowski View author publications You can also search for this author inPubMed Google Scholar * John Ravits View author publications You can also search for
this author inPubMed Google Scholar * Teepu Siddique View author publications You can also search for this author inPubMed Google Scholar * Jerry B Lingrel View author publications You can
also search for this author inPubMed Google Scholar * Janice Robertson View author publications You can also search for this author inPubMed Google Scholar * Hanno Steen View author
publications You can also search for this author inPubMed Google Scholar * Azad Bonni View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.B.
directed and coordinated the project. G.G. designed and performed or participated in all experiments. J.B. performed mouse husbandry and survival studies. J.B.L. provided α2-Na/K ATPase
knockout mice. H.S. performed mass spectrometry analysis. J. Ravits, T.S. and J. Robertson provided human tissue samples. The manuscript was written by G.G. and A.B. and commented on by all
authors. CORRESPONDING AUTHOR Correspondence to Azad Bonni. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. INTEGRATED SUPPLEMENTARY INFORMATION
SUPPLEMENTARY FIGURE 1 Α-ADDUCIN IN SPINAL CORD IS UPREGULATED IN SYMPTOMATIC SOD1G93A SPINAL CORD WITHIN ASTROCYTES. (A) Immunoblots for α-Adducin protein at 60 and 90 day old SOD1G93A
mice show α-Adducin is upregulated at 90 days. (B) Immunohistochemistry with in sections of the lumbar spinal cord from symptomatic SOD1G93A mice displays Ser436-phosphorylated α-Adducin
does not co-localize with the motor neuron marker (SMi32). Arrowheads indicate motor neurons; scale bar 50μm. (C) Immunohistochemistry from sections of control wild type lumbar spinal cord
displays Ser436-phosphorylated α-Adducin does not co-localize with the motor neuron marker (SMi32); _upper panels_. Arrowheads indicate motor neurons. Immunohistochemistry from sections of
control wild type lumbar spinal cord displays Ser436-phosphorylated α-Adducin co-localize with the astrocyte marker (GFAP) lower panel_s_. Scale bar 50μm. (A) are cropped; full length images
are presented in Supplementary Figure 11. SUPPLEMENTARY FIGURE 2 EXPRESSION OF A RNAI-RESISTANT FORM OF Α-ADDUCIN IN SOD1G93A ASTROCYTES RESTORES THE ABILITY OF SOD1G93A ASTROCYTES TO
INDUCE NON-CELL AUTONOMOUS MOTOR NEURON CELL DEATH. (A) Knockdown of α-Adducin relative to control U6 in astrocytes (B) Co-cultured astrocytes and motor neurons were subjected to
immunocytochemistry with the motor neuron nuclear protein Islet1 (red) and the dendrite protein MAP2 (green); scale bar 50μm. Wild type astrocytes transfected with the control U6 or
α-Adducin RNAi plasmid had little or no effect on motor primary motor neurons cell death or dendrite abnormalities (_upper left panel)_; quantified (C and D). Control U6 SOD1G93A astrocytes
induced non-cell autonomous motor neuron cell death and dendrite abnormalities (_upper right panel_); quantified (C and D). Knockdown of α-Adducin in SOD1G93A astrocytes protected motor
neurons against the non-cell autonomous cell death and dendrite abnormalities (_lower left panel_); quantified (C AND D). Expressions of an RNAi-resistant form of α-Adducin (Add-Res) in the
background of α-Adducin RNAi in SOD1G93A astrocytes restored the ability of the SOD1G93A astrocytes to induce non-cell autonomous cell death and dendrite abnormalities in motor neurons
(_lower right panel_); quantified (D and E). All data in bar charts show mean ± s.e.m (***p<0.001; unpaired t-test). (A) are cropped; full length images are presented in Supplementary
Figure 11. SUPPLEMENTARY FIGURE 3 LENTIVIRIAL MEDIATED KNOCKDOWN _IN VIVO_ PREDOMINATELY TARGET ASTROCYTES. (A) Immunohistochemistry with GFP in sections of the lumbar spinal cord from
SOD1G93A mice displaying percent GFP positive astrocytes (GFAP), microglia (Iba1) and motor neurons (SMi32) 30 days post-injection. Arrowheads indicate motor neurons; scale bar 50μm. (B)
Quantifications of percent GFP positive cells revealed lentivirus predominately target astrocytes; n=~300 per cell type/three. All data in bar charts show ± s.e.m (***p<0.001; ANOVA). (C)
Immunohistochemistry with GFP in sections of the lumbar spinal cord from wild type mice displaying percent GFP positive astrocytes (GFAP), microglia (Iba1) and motor neurons (SMi32) 30 days
post-injection. Arrowheads indicate motor neurons; scale bar 50μm. (D) Quantifications of percent GFP positive cells revealed lentivirus predominately target astrocytes; n=~300 per cell
type/three. All data in bar charts show ± s.e.m (***p<0.001; unpaired t-test). SUPPLEMENTARY FIGURE 4 KNOCKDOWN OF Α-ADDUCIN IN SOD1G93A MICE DECREASES IMMUNOREACTIVITY FOR PHOSPHORYLATED
SER436-Α-ADDUCIN. Spinal cord from SOD1G93A mice injected intraspinally with lentivirus expressing short hairpin RNAs targeting α-Adducin and encoding GFP (LV-Addi) or the corresponding
control U6 (LV-U6) were subjected to immunohistochemistry using GFP and phospho-α-Adducin (red) antibodies. Knockdown of α-Adducin (LV-Addi) led to a decreased in immunoreactivity of
phospho-α-Adducin within the GFP-labeled ventral horn as compared to control U6 (LV-U6) injected ventral horn; scale bar 50μm. SUPPLEMENTARY FIGURE 5 KNOCKDOWN OF Α-ADDUCIN OR Α2-NA/K ATPASE
IN SOD1G93A MICE DO NOT ALTER GLIOSIS IN THE SPINAL CORD. Spinal cord from SOD1G93A mice injected intraspinally with lentivirus expressing short hairpin RNAs targeting α-Adducin or α2-Na/K
ATPase or the corresponding control U6 virus were subjected to immunohistochemistry using GFP and the GFAP (red) antibodies. Knockdown of α-Adducin (LV-Addi) or α2-Na/K ATPase (LV-ATPi) had
little or no effect on the presence or abundance of astrocytes within the GFP-labeled ventral horn; scale bar 50μm. SUPPLEMENTARY FIGURE 6 KNOCKDOWN OF Α-ADDUCIN OR Α2-NA/K ATPASE IN
SOD1G93A MICE DO NOT ALTER MICROGLIOSIS IN SPINAL CORD. Spinal cord from SOD1G93A mice injected intraspinally with lentivirus expressing short hairpin RNAs targeting α-Adducin or α2-Na/K
ATPase or the corresponding control U6 virus were subjected to immunohistochemistry using GFP and the Iba1 (red) antibodies. Knockdown of α-Adducin (LV-Addi) or α2-Na/K ATPase (LV-ATPi) had
little or no effect on the presence or abundance of microglia within the GFP-labeled ventral horn; scale bar 50μm. SUPPLEMENTARY FIGURE 7 Α2-NA/K ATPASE CO-IMMUNOPRECIPITATES WITH Α-ADDUCIN
IN SPINAL CORD LYSATES AND IS SPECIFICALLY UPREGULATED IN ASTROCYTES IN SYMPTOMATIC SOD1G93A MICE. (A) Immunoblots show immunoprecipitated α-Adducin from SOD1G93A and control wild type
spinal cord lysates subjected to immunoblotting with the α-Adducin and α2-Na/K ATPase antibodies following glycine elution, confirming α2-Na/K ATPase as an interactor of α-Adducin (_left
panels)_. Immunoblots show α2-Na/K ATPase is predominately expressed in primary glial cultures relative to primary motor neuron cultures enriched with the neuron marker β-tubulin. 14-3-3β is
used as an internal control (_right panel_). (B) Immunohistochemistry with astrocyte marker GFAP and α2-Na/K ATPase antibody in sections of the lumbar spinal cord from SOD1G93A mice at 60
days displays α2-Na/K ATPase expression within astrocytes; scale bar 50μm. (C) Immunohistochemistry with GFAP and α2-Na/K ATPase antibody in sections of the lumbar spinal cord from
symptomatic SOD1G93A mice at 120 days displays upregulation of α2-Na/K ATPase expression within astrocytes; scale bar 50μm. (A) are cropped; full length images are presented in Supplementary
Figure 11. SUPPLEMENTARY FIGURE 8 INTRASPINALLY INJECTION OF CONTROL LENTIVIRUS IN SOD1G93A MICE HAD NO EFFECT ON MOTOR NEURON SURVIVAL. (A) Spinal cord from end stage SOD1G93A mice
injected at age 90 days intraspinally with the control lentivirus encoding GFP (LV-U6 SOD1G93A) was subjected to immunohistochemistry at end stage. End stage was defined as a time point at
which the animal was unable to upright itself within 30s of placement on its side. Immunohistochemistry with GFP in SOD1G93A lumbar sections revealed delivery of control injected virus
(LV-U6) into the ventral horn; scale bar 100μm. Alternating GFP positive sections were subjected to immunohistochemistry using the GFP antibody and the neurofilment-SMi32 antibody (red), a
motor neuron marker, or Nissl stained (_lower panels_) for quantification of surviving motor neurons within GFP-labeled injected ventral horn and contralateral non-injected ventral horn
(n≥20 sections per animal); scale bar 50μm. Control LV-U6 SOD1G93A mice (n=3) displayed equivalent degeneration of motor neurons within injected GFP-labeled ventral horn and non-injected
contralateral ventral horn. Arrowheads indicate surviving motor neurons; quantification shown in (B). SUPPLEMENTARY FIGURE 9 HETEROZYGOUS DISRUPTION OF THE Α2-NA/K ATPASE GENE IN SOD1G93A
MICE DELAYS MOTOR NEURON DEGENERATION. (A) Nissl stained sections from endstage control SOD1G93A mice (n=5) and aged-matched SOD1G93A littermates heterozygous-null for the α2-Na/K ATPase
allele (n=5) displayed more than twice the number of motor neurons in ATPase+/-;SOD1G93A than control SOD1G93A mice. Arrow heads indicate surviving motor neurons; quantification shown in
(B); scale bar 50μm (***p<0.001; unpaired t-test). SUPPLEMENTARY FIGURE 10 CONDITION MEDIA FROM HETEROZYGOUS-NULL FROM Α2-NA/K ATPASE SOD1G93A ASTROCYTES IS NEUROPROTECTIVE. (A)
Precondition media from wild type, SOD1G93A, and heterozygous-null α2-Na/K ATPase; SOD1G93A astrocytes were exposed to motor neurons and subjected to immunocytochemistry with antibodies
recognizing the motor neuron nuclear protein Islet1 (red) and the dendrite protein MAP2 (green); scale bar 50μm. Precondition media from wild type astrocytes had little or no effect on motor
neuron survival (_upper panels_); quantified (B). Preconditioned medium from SOD1G93A astrocytes induced non-cell autonomous motor neuron cell death (_middle_); quantified (B).
Preconditioned medium from heterozygous-null α2-Na/K ATPase; SOD1G93A astrocytes protected motor neurons against the non-cell autonomous cell death (_lower panel_); All data in bar charts
show mean ± s.e.m (***p<0.001; unpaired t-test). SUPPLEMENTARY FIGURE 11 FULL SCANS OF KEY WESTERN BLOT DATA. IN MANY EXPERIMENTS, MEMBRANES WERE STRIPPED AND REBLOTTED WITH A SECOND
ANTIBODY. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES Supplementary Figures 1–11 (PDF 11653 kb) SUPPLEMENTARY METHODS CHECKLIST (PDF 1629 kb) SOD1G93A MICE AT ENDSTAGE. (MPG
40096 KB) HETEROZYGOUS-NULL Α2-NA/K ATPASE+/-; SOD1G93A AGE MATCHED LITTERMATES (MPG 17088 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gallardo,
G., Barowski, J., Ravits, J. _et al._ An α2-Na/K ATPase/α-adducin complex in astrocytes triggers non–cell autonomous neurodegeneration. _Nat Neurosci_ 17, 1710–1719 (2014).
https://doi.org/10.1038/nn.3853 Download citation * Received: 21 July 2014 * Accepted: 29 September 2014 * Published: 26 October 2014 * Issue Date: December 2014 * DOI:
https://doi.org/10.1038/nn.3853 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
