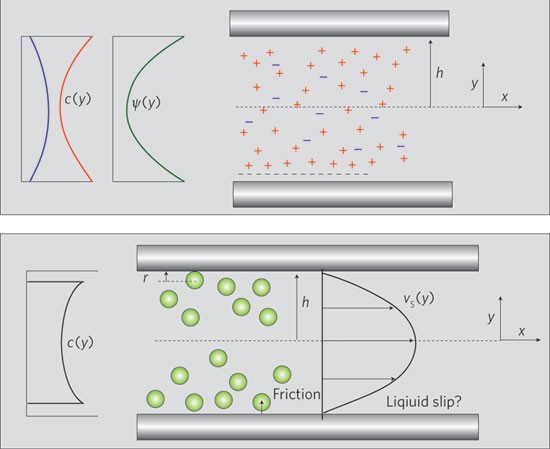
Principles and applications of nanofluidic transport
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The evolution from microfluidic to nanofluidic systems has been accompanied by the emergence of new fluid phenomena and the potential for new nanofluidic devices. This review
provides an introduction to the theory of nanofluidic transport, focusing on the various forces that influence the movement of both solvents and solutes through nanochannels, and reviews the
applications of nanofluidic devices in separation science and energy conversion. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS TOWARD SUB-SECOND SOLUTION EXCHANGE DYNAMICS IN FLOW REACTORS FOR
LIQUID-PHASE TRANSMISSION ELECTRON MICROSCOPY Article Open access 21 March 2024 EVAPORATION-DRIVEN TRANSPORT-CONTROL OF SMALL MOLECULES ALONG NANOSLITS Article Open access 26 February 2021
NANOFLUIDICS Article 19 September 2024 REFERENCES * Turner, S. W., Perez, A. M., Lopez, A. & Craighead, H. G. Monolithic nanofluid sieving structures for DNA manipulation. _J. Vac. Sci.
Technol. B_ 16, 3835–3840 (1998). CAS Google Scholar * Eijkel, J. C. T. & van den Berg, A. Nanofluidics: what is it and what can we expect from it? _Microfluid. Nanofluid._ 1, 249–267
(2005). CAS Google Scholar * Abgrall, P. & Nguyen, N. T. Nanofluidic devices and their applications. _Anal. Chem._ 80, 2326–2341 (2008). CAS Google Scholar * Mijatovic, D., Eijkel,
J. C. T. & van den Berg, A. Technologies for nanofluidic systems: top-down vs. bottom-up — a review. _Lab Chip_ 5, 492–500 (2005). CAS Google Scholar * Perry, J. L. & Kandlikar, S.
G. Review of fabrication of nanochannels for single phase liquid flow. _Microfluid. Nanofluid._ 2, 185–193 (2006). CAS Google Scholar * Schoch, R. B., Han, J. Y. & Renaud, P.
Transport phenomena in nanofluidics. _Rev. Mod. Phys._ 80, 839–883 (2008). CAS Google Scholar * Yuan, Z., Garcia, A. L., Lopez, G. P. & Petsev, D. N. Electrokinetic transport and
separations in fluidic nanochannels. _Electrophoresis_ 28, 595–610 (2007). CAS Google Scholar * Succi, S., Mohammad, A. A. & Horbach, J. Lattice-Boltzmann simulation of dense
nanoflows: A comparison with molecular dynamics and Navier-Stokes solutions. _Int. J. Mod. Phys. C_ 18, 667–675 (2007). CAS Google Scholar * Wijmans, J. G. & Baker, R. W. The
solution-diffusion model — a review. _J. Membr. Sci._ 107, 1–21 (1995). CAS Google Scholar * Gad-el-Hak, M. The fluid mechanics of microdevices — The Freeman scholar lecture. _J. Fluid
Eng.-T. ASME_ 121, 5–33 (1999). Google Scholar * Deen, W. M. Hindered transport of large molecules in liquid-filled pores. _AlChE J._ 33, 1409–1425 (1987). CAS Google Scholar * Prieve, D.
C. & Hoysan, P. M. Role of colloidal forces in hydrodynamic chromatography. _J. Colloid Interf. Sci._ 64, 201–213 (1978). CAS Google Scholar * Ruckenstein, E. & Prieve, D. C.
Adsorption and desorption of particles and their chromatographic separation. _AlChE J._ 22, 276–283 (1976). CAS Google Scholar * Bowen, W. R. & Mukhtar, H. Characterisation and
prediction of separation performance of nanofiltration membranes. _J. Membr. Sci._ 112, 263–274 (1996). CAS Google Scholar * Lyklema, J. _Fundamentals of Interface and Colloid Science,
Fundamentals_ 1st edn (Academic Press, 2000). Google Scholar * Christenson, H. K. & Claesson, P. M. Direct measurements of the force between hydrophobic surfaces in water. _Ad. Colloid
Interf. Sci._ 91, 391–436 (2001). CAS Google Scholar * Meyer, E. E., Rosenberg, K. J. & Israelachvili, J. Recent progress in understanding hydrophobic interactions. _Proc. Natl Acad.
Sci. USA_ 103, 15739–15746 (2006). CAS Google Scholar * Norde, W. in _Physical Chemistry of Biological Interfaces_ (ed. Dekker, M.) 115–136 (CRC, 2000). Google Scholar * Giddings, J. C.,
Kucera, E., Russell, C. P. & Myers, M. N. Statistical theory for equilibrium distribution of rigid molecules in inert porous networks. Exclusion chromatography. _J. Phys. Chem._ 72,
4397–4408 (1968). CAS Google Scholar * Teraoka, I. Polymer solutions in confining geometries. _Prog. Polym. Sci._ 21, 89–149 (1996). CAS Google Scholar * Burgreen, D. & Nakache, F.
R. Electrokinetic flow in ultrafine capillary slits. _J. Phys. Chem._ 68, 1084–1091 (1964). Google Scholar * Levine, S., Marriott, J. R., Neale, G. & Epstein, N. Theory of
electrokinetic flow in fine cylindrical capillaries at high zeta-potentials. _J. Colloid Interf. Sci._ 52, 136–149 (1975). Google Scholar * Vinogradova, O. I. Slippage of water over
hydrophobic surfaces. _Int. J. Miner. Process._ 56, 31–60 (1999). CAS Google Scholar * Cottin-Bizonne, C., Cross, B., Steinberger, A. & Charlaix, E. Boundary slip on smooth hydrophobic
surfaces: Intrinsic effects and possible artefacts. _Phys. Rev. Lett._ 94, 056102 (2005). CAS Google Scholar * Muller, V. M., Sergeeva, I. P., Sobolev, V. D. & Churaev, N. V. Boundary
effects in the theory of electrokinetic phenomena. _Colloid J. USSR_ 48, 606–614 (1986). Google Scholar * Bouzigues, C. I., Tabeling, P. & Bocquet, L. Nanofluidics in the Debye layer
at hydrophilic and hydrophobic surfaces. _Phys. Rev. Lett._ 101, 114503 (2008). CAS Google Scholar * Joly, L., Ybert, C., Trizac, E. & Bocquet, L. Liquid friction on charged surfaces:
From hydrodynamic slippage to electrokinetics. _J. Chem. Phys._ 125, 204716 (2006). Google Scholar * Vermesh, U. et al. Fast nonlinear ion transport via field-induced hydrodynamic slip in
sub-20-nm hydrophilic nanofluidic transistors. _Nano Lett._ 9, 1315–1319 (2009). CAS Google Scholar * Ren, Y. Q. & Stein, D. Slip-enhanced electrokinetic energy conversion in
nanofluidic channels. _Nanotechnology_ 19, 195707 (2008). Google Scholar * van der Heyden, F. H. J., Bonthuis, D. J., Stein, D., Meyer, C. & Dekker, C. Power generation by
pressure-driven transport of ions in nanofluidic channels. _Nano Lett._ 7, 1022–1025 (2007). CAS Google Scholar * Daiguji, H., Yang, P. D., Szeri, A. J. & Majumdar, A.
Electrochemomechanical energy conversion in nanofluidic channels. _Nano Lett._ 4, 2315–2321 (2004). CAS Google Scholar * Stein, D., van der Heyden, F. H. J., Koopmans, W. J. A. &
Dekker, C. Pressure-driven transport of confined DNA polymers in fluidic channels. _Proc. Natl Acad. Sci USA_ 103, 15853–15858 (2006). CAS Google Scholar * Holt, J. K. et al. Fast mass
transport through sub-2-nanometer carbon nanotubes. _Science_ 312, 1034–1037 (2006). CAS Google Scholar * Majumder, M., Chopra, N., Andrews, R. & Hinds, B. J. Nanoscale hydrodynamics:
Enhanced flow in carbon nanotubes. _Nature_ 438, 44–44 (2005). CAS Google Scholar * Eijkel, J. C. T., Bomer, J. G. & van den Berg, A. Osmosis and pervaporation in polyimide submicron
microfluidic channel structures. _Appl. Phys. Lett._ 87, 114103 (2005). Google Scholar * Karlsson, R. et al. Moving-wall-driven flows in nanofluidic systems. _Langmuir_ 18, 4186–4190
(2002). CAS Google Scholar * Soare, M. A., Picu, R. C., Tichy, J., Lu, T. M. & Wang, G. C. Fluid transport through nanochannels using nanoelectromechanical actuators. _J. Intell.
Mater. Sys. Struct._ 17, 231–238 (2006). Google Scholar * Tas, N. R., Berenschot, J. W., Lammerink, T. S. J., Elwenspoek, M. & van den Berg, A. Nanofluidic bubble pump using surface
tension directed gas injection. _Anal. Chem._ 74, 2224–2227 (2002). CAS Google Scholar * Prieve, D. C., Anderson, J. L., Ebel, J. P. & Lowell, M. E. Motion of a particle generated by
chemical gradients. 2. Electrolytes. _J. Fluid Mech._ 148, 247–269 (1984). CAS Google Scholar * Anderson, J. L., Prieve, D. C. & Ebel, J. P. Chemically induced migration of particles
across fluid streamlines. _Chem. Eng. Commun._ 55, 211–224 (1987). CAS Google Scholar * Keh, H. J. & Wei, Y. K. Diffusioosmosis and electroosmosis of electrolyte solutions in fibrous
porous media. _J. Colloid Interf. Sci._ 252, 354–364 (2002). CAS Google Scholar * Qian, S. Z., Das, B. & Luo, X. B. Diffusioosmotic flows in slit nanochannels. _J. Colloid Interf.
Sci._ 315, 721–730 (2007). CAS Google Scholar * Ajdari, A. & Bocquet, L. Giant amplification of interfacially driven transport by hydrodynamic slip: Diffusio-osmosis and beyond. _Phys.
Rev. Lett._ 96, 186102 (2006). Google Scholar * Goedecke, N., Eijkel, J. & Manz, A. Evaporation driven pumping for chromatography application. _Lab Chip_ 2, 219–223 (2002). CAS Google
Scholar * Wheeler, T. D. & Stroock, A. D. The transpiration of water at negative pressures in a synthetic tree. _Nature_ 455, 208–212 (2008). CAS Google Scholar * Huh, D. et al.
Tuneable elastomeric nanochannels for nanofluidic manipulation. _Nature Mater._ 6, 424–428 (2007). CAS Google Scholar * Quake, S. R. & Scherer, A. From micro- to nanofabrication with
soft materials. _Science_ 290, 1536–1540 (2000). CAS Google Scholar * Poppe, H. Some reflections on speed and efficiency of modern chromatographic methods. _J. Chromatogr. A_ 778, 3–21
(1997). CAS Google Scholar * Kievsky, Y. Y. et al. Dynamics of molecular diffusion of rhodamine 6G in silica nanochannels. _J. Chem. Phys._ 128, 151102 (2008). CAS Google Scholar *
Durand, N. F. Y., Bertsch, A., Todorova, M. & Renaud, P. Direct measurement of effective diffusion coefficients in nanochannels using steady-state dispersion effects. _Appl. Phys. Lett._
91, 203106 (2007). Google Scholar * Karnik, R., Castelino, K., Duan, C. H. & Majumdar, A. Diffusion-limited patterning of molecules in nanofluidic channels. _Nano Lett._ 6, 1735–1740
(2006). CAS Google Scholar * Yamaguchi, A. et al. Diffusion of metal complexes inside of silica-surfactant nanochannels within a porous alumina membrane. _J. Phys. Chem. B_ 112, 2024–2030
(2008). CAS Google Scholar * Yamaguchi, A., Yoda, T., Suzuki, S., Morita, K. & Teramae, N. Diffusivities of tris(2,2′-bipyridyl)ruthenium inside silica-nanochannels modified with
alkylsilanes. _Anal. Sci._ 22, 1501–1507 (2006). CAS Google Scholar * Schoch, R. B., Bertsch, A. & Renaud, P. pH-controlled diffusion of proteins with different pl values across a
nanochannel on a chip. _Nano Lett._ 6, 543–547 (2006). CAS Google Scholar * Crank, J. _The Mathematics of Diffusion_ (Oxford Univ. Press, 1975). Google Scholar * Delamarche, E., Bernard,
A., Schmid, H., Michel, B. & Biebuyck, H. Patterned delivery of immunoglobulins to surfaces using microfluidic networks. _Science_ 276, 779–781 (1997). CAS Google Scholar * Pennathur,
S. & Santiago, J. G. Electrokinetic transport in nanochannels. 1. Theory. _Anal. Chem._ 77, 6772–6781 (2005). CAS Google Scholar * Pennathur, S. & Santiago, J. G. Electrokinetic
transport in nanochannels. 2. Experiments. _Anal. Chem._ 77, 6782–6789 (2005). CAS Google Scholar * Pennathur, S. et al. Free-solution oligonucleotide separation in nanoscale channels.
_Anal. Chem._ 79, 8316–8322 (2007). CAS Google Scholar * Xuan, X. C. Ion separation in nanofluidics. _Electrophoresis_ 29, 3737–3743 (2008). CAS Google Scholar * Wang, X. Y., Kang, J.
Z., Wang, S. L., Lu, J. J. & Liu, S. R. Chromatographic separations in a nanocapillary under pressure-driven conditions. _J. Chromatogr. A_ 1200, 108–113 (2008). CAS Google Scholar *
Ogston, A. G. The spaces in a uniform random suspension of fibres. _Trans. Faraday Soc._ 54, 1754–1757 (1958). Google Scholar * Fu, J. P., Schoch, R. B., Stevens, A. L., Tannenbaum, S. R.
& Han, J. Y. A patterned anisotropic nanofluidic sieving structure for continuous-flow separation of DNA and proteins. _Nature Nanotech._ 2, 121–128 (2007). CAS Google Scholar * Blom,
M. T., Chmela, E., Oosterbroek, R. E., Tijssen, R. & van den Berg, A. On-chip hydrodynamic chromatography separation and detection of nanoparticles and biomolecules. _Anal. Chem._ 75,
6761–6768 (2003). CAS Google Scholar * Stein, D., van der Heyden, F. H. J., Koopmans, W. J. A. & Dekker, C. Pressure driven transport iof confined DNA polymers in fluidic channels.
_Proc. Natl Acad. Sci. USA_ 103, 15853–15858 (2006). CAS Google Scholar * Han, J. & Craighead, H. G. Separation of long DNA molecules in a microfabricated entropic trap array.
_Science_ 288, 1026–1029 (2000). CAS Google Scholar * Li, Z. R. et al. Continuum transport model of Ogston sieving in patterned nanofilter arrays for separation of rod-like biomolecules.
_Electrophoresis_ 29, 329–339 (2008). Google Scholar * Turner, S. W. P., Cabodi, M. & Craighead, H. G. Confinement-induced entropic recoil of single DNA molecules in a nanofluidic
structure. _Phys. Rev. Lett._ 88, 128103 (2002). CAS Google Scholar * Austin, R. Nanofluidics: A fork in the nano-road. _Nature Nanotech._ 2, 79–80 (2007). CAS Google Scholar *
Tegenfeldt, J. O. et al. Micro- and nanofluidics for DNA analysis. _Anal. Bioanal. Chem._ 378, 1678–1692 (2004). CAS Google Scholar * Mannion, J. T., Reccius, C. H., Cross, J. D. &
Craighead, H. G. Conformational analysis of single DNA molecules undergoing entropically induced motion in nanochannels. _Biophys. J._ 90, 4538–4545 (2006). CAS Google Scholar * Cross, J.
D., Strychalski, E. A. & Craighead, H. G. Size-dependent DNA mobility in nanochannels. _J. Appl. Phys._ 102, 024514 (2007). Google Scholar * Salieb-Beugelaar, G. B. et al.
Field-dependent DNA mobility in 20 nm high nanoslits. _Nano Lett._ 8, 1785–1790 (2008). CAS Google Scholar * Kuo, T. C., Sloan, L. A., Sweedler, J. V. & Bohn, P. W. Manipulating
molecular transport through nanoporous membranes by control of electrokinetic flow: Effect of surface charge density and Debye length. _Langmuir_ 17, 6298–6303 (2001). CAS Google Scholar *
Karnik, R., Castelino, K. & Majumdar, A. Field-effect control of protein transport in a nanofluidic transistor circuit. _Appl. Phys. Lett._ 88, 123114 (2006). Google Scholar * Miedema,
H. et al. A biological porin engineered into a molecular, nanofluidic diode. _Nano Lett._ 7, 2886–2891 (2007). CAS Google Scholar * Garcia-Gimenez, E., Alcaraz, A., Aguilella, V. M. &
Ramirez, P. Directional ion selectivity in a biological nanopore with bipolar structure. _J. Membr. Sci._ 331, 137–142 (2009). CAS Google Scholar * Ali, M., Ramirez, P., Mafe, S.,
Neumann, R. & Ensinger, W. A pH-tunable nanofluidic diode with a broad range of rectifying properties. _ACS Nano_ 3, 603–608 (2009). CAS Google Scholar * Karnik, R., Duan, C. H.,
Castelino, K., Daiguji, H. & Majumdar, A. Rectification of ionic current in a nanofluidic diode. _Nano Lett._ 7, 547–551 (2007). CAS Google Scholar * Alcaraz, A. et al. A pH-tunable
nanofluidic diode: Electrochemical rectification in a reconstituted single ion channel. _J. Phys. Chem. B_ 110, 21205–21209 (2006). CAS Google Scholar * Cheng, L. J. & Guo, L. J.
Rectified ion transport through concentration gradient in homogeneous silica nanochannels. _Nano Lett._ 7, 3165–3171 (2007). CAS Google Scholar * Fan, R., Huh, S., Yan, R., Arnold, J.
& Yang, P. D. Gated proton transport in aligned mesoporous silica films. _Nature Mater._ 7, 303–307 (2008). CAS Google Scholar * Fan, R., Yue, M., Karnik, R., Majumdar, A. & Yang,
P. D. Polarity switching and transient responses in single nanotube nanofluidic transistors. _Phys. Rev. Lett._ 95, 086607 (2005). Google Scholar * Karnik, R. et al. Electrostatic control
of ions and molecules in nanofluidic transistors. _Nano Lett._ 5, 943–948 (2005). CAS Google Scholar * Kuo, T. C. et al. Gateable nanofluidic interconnects for multilayered microfluidic
separation systems. _Anal. Chem._ 75, 1861–1867 (2003). CAS Google Scholar * Vlassiouk, I. & Siwy, Z. S. Nanofluidic diode. _Nano Lett._ 7, 552–556 (2007). CAS Google Scholar * Gijs,
M. A. M. Device physics: Will fluidic electronics take off? _Nature Nanotech._ 2, 268–270 (2007). CAS Google Scholar * Pu, Q. S., Yun, J. S., Temkin, H. & Liu, S. R. Ion-enrichment
and ion-depletion effect of nanochannel structures. _Nano Lett._ 4, 1099–1103 (2004). CAS Google Scholar * Wang, Y. C., Stevens, A. L. & Han, J. Y. Million-fold preconcentration of
proteins and peptides by nanofluidic filter. _Anal. Chem._ 77, 4293–4299 (2005). CAS Google Scholar * Mani, A., Zangle, T. A. & Santiago, J. G. On the propagation of concentration
polarization from microchannel-nanochannel interfaces part I: Analytical model and characteristic analysis. _Langmuir_ 25, 3898–3908 (2009). CAS Google Scholar * Zangle, T. A., Mani, A.
& Santiago, J. G. On the propagation of concentration polarization from microchannel-nanochannel interfaces part II: Numerical and experimental study. _Langmuir_ 25, 3909–3916 (2009).
CAS Google Scholar * Plecis, A., Nanteuil, C., Haghiri-Gosnet, A. M. & Chen, Y. Electropreconcentration with charge-selective nanochannels. _Anal. Chem._ 80, 9542–9550 (2008). CAS
Google Scholar * Han, A. P., de Rooij, N. F. & Staufer, U. Design and fabrication of nanofluidic devices by surface micromachining. _Nanotechnology_ 17, 2498–2503 (2006). CAS Google
Scholar * Han, J. Y., Fu, J. P. & Schoch, R. B. Molecular sieving using nanofilters: Past, present and future. _Lab Chip_ 8, 23–33 (2008). CAS Google Scholar * Eijkel, J. C. T.
Scaling revisited. _Lab Chip_ 7, 1630–1632 (2007). CAS Google Scholar * Osterle, J. F. Unified treatment of thermodynamics of steady-state energy conversion. _Appl. Sci. Res. A_ 12,
425–434 (1964). Google Scholar * Burgreen, D. & Nakache, F. R. Efficiency of pumping and power generation in ultrafine electrokinetic systems _J. Appl. Mech._ 32, 675–679 (1965). Google
Scholar * Davidson, C. & Xuan, X. C. Electrokinetic energy conversion in slip nanochannels. _J. Power Sources_ 179, 297–300 (2008). CAS Google Scholar * Davidson, C. & Xuan, X.
C. Effects of Stern layer conductance on electrokinetic energy conversion in nanofluidic channels. _Electrophoresis_ 29, 1125–1130 (2008). CAS Google Scholar * Pennathur, S., Eijkel, J. C.
T. & van den Berg, A. Energy conversion in microsystems: is there a role for micro/nanofluidics? _Lab Chip_ 7, 1234–1237 (2007). Google Scholar * Probstein, R. F. _Physicochemical
Hydrodynamics: An Introduction_ 2nd edn (Wiley, 1994). Google Scholar * van Oss, C. J. Long-range and short-range mechanisms of hydrophobic attraction and hydrophilic repulsion in specific
and aspecific interactions. _J. Mol. Recogn._ 16, 177–190 (2003). CAS Google Scholar * Ninham, B. W. & Yaminsky, V. Ion binding and ion specificity: The Hofmeister effect and Onsager
and Lifshitz theories. _Langmuir_ 13, 2097–2108 (1997). CAS Google Scholar * Pierret, R. F. _Semiconductor Device Fundamentals_ (Addison-Wesley, 1996). Google Scholar Download references
ACKNOWLEDGEMENTS We thank the Dutch Technology Foundation (STW) for financial support via a NanoNed grant (TMM 7128). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * BIOS/Lab on a Chip group,
MESA+ Institute for Nanotechnology, University of Twente, PO Box 217, 7500 AE Enschede, The Netherlands., W. Sparreboom, A. van den Berg & J. C. T. Eijkel Authors * W. Sparreboom View
author publications You can also search for this author inPubMed Google Scholar * A. van den Berg View author publications You can also search for this author inPubMed Google Scholar * J. C.
T. Eijkel View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to W. Sparreboom. RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sparreboom, W., van den Berg, A. & Eijkel, J. Principles and applications of nanofluidic transport. _Nature Nanotech_ 4, 713–720 (2009).
https://doi.org/10.1038/nnano.2009.332 Download citation * Published: 08 November 2009 * Issue Date: November 2009 * DOI: https://doi.org/10.1038/nnano.2009.332 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative
