In vivo calcium imaging to illuminate neurocircuit activity dynamics underlying naturalistic behavior
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

The brain perceives the outside world, coordinates behavior, regulates emotional states, and forms memories about these experiences. To orchestrate such a diverse array of functions,
billions of neurons interact to form circuits with the capacity to compute sensory information and rapidly generate appropriate behavior. Understanding these processes and how the
dysregulation of circuits contributes to psychiatric illness is a major goal of neuroscience research and has led to the development of novel tools for circuit-level analysis of the brain in
behaving animals (Jennings and Stuber, 2014). One approach for monitoring circuit-specific activity dynamics is _in vivo_ calcium imaging. During periods of increased neural activity,
dynamic fluctuations in calcium levels correlate with events such as action potential generation, exocytosis of neurotransmitters, changes in synaptic plasticity, and gene transcription.
Thus, monitoring calcium dynamics with genetically encoded calcium indicators, such as GCaMP6, can serve as a proxy for neural activity within defined neurocircuits (Chen et al, 2013).
Imaging of neural activity using calcium indicators has largely been accomplished with _in vivo_ two-photon microscopy, which provides unprecedented cellular and subcellular spatial
resolution; however, two-photon imaging must be performed in head-fixed animals, greatly limiting the assessment of naturalistic behavior (Svoboda and Yasuda, 2006). To circumvent these
limitations, new methods for visualizing and quantifying calcium-mediated fluorescent signals have been developed for use in freely moving animals. Visualizing calcium transients in freely
moving mice requires imaging devices that are small enough to fit on an animal’s head and light enough to be carried by the animal. In the last few years, two such approaches have been
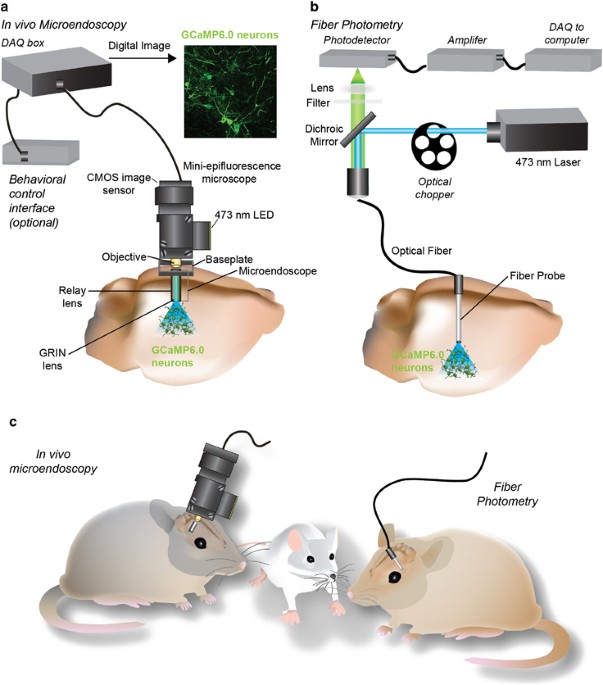
developed: mini-epifluorescence microscopes used in conjunction with gradient index (GRIN) lens microendoscopes (Ziv et al, 2013) and fiber photometry (Cui et al, 2013; Gunaydin et al, 2014)
(Figure 1). Mini-epifluorescence microscopes are ideal for measuring somatic calcium activity dynamics. Importantly, with this novel technology, the activity of hundreds of genetically and
spatially defined neurons within a single animal can be repeatedly imaged, permitting the analysis of cellular network dynamics that orchestrate behavior as well as the transition to
pathological disease states (Ziv et al, 2013). In addition, both microendoscopic imaging and fiber photometry are capable of recording neural activity many millimeters deep within the brain.
Fiber photometry utilizes optical fibers to detect bulk changes in calcium-mediated fluorescence in the soma (Cui et al, 2013) or terminal fields (Gunaydin et al, 2014) of genetically
defined neurons. While fiber photometry lacks cellular resolution, it can provide important insight into the synchronous activity dynamics within a defined neurocircuit during both adaptive
and aberrant behavioral states. Importantly, the light weight and small size of both these devices allows them to be coupled with a wide array of behavioral paradigms, such as sensory tasks,
elevated plus maze for measures of anxiety, tail suspension for research related to depression, as well as complex learning paradigms utilizing operant chambers. Although advances are
continuously being made in all aspects of these devices, current models of the mini-epifluorescence microscopes are not yet compatible with behavioral paradigms where mice are submerged in
water, such as the forced swim test. Nonetheless, these innovative approaches can elucidate the temporal and spatial activity patterns of molecularly defined neurocircuits during a multitude
of adaptive and maladaptive behaviors. FUNDING AND DISCLOSURE G.D. Stuber has received prototype versions of a mini-epifluorescence microscope system from Inscopix Inc. for testing
purposes. The authors declare no conflict of interest. REFERENCES * Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A _et al_ (2013). Ultrasensitive fluorescent proteins for
imaging neuronal activity. _Nature_ 499: 295–300. Article CAS Google Scholar * Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM _et al_ (2013). Concurrent activation of striatal
direct and indirect pathways during action initiation. _Nature_ 494: 238–242. Article CAS Google Scholar * Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A _et
al_ (2014). Natural neural projection dynamics underlying social behavior. _Cell_ 157: 1535–1551. Article CAS Google Scholar * Jennings JH, Stuber GD (2014). Tools for resolving
functional activity and connectivity within intact neural circuits. _Curr Biol_ 24: R41–R50. Article CAS Google Scholar * Svoboda K, Yasuda R (2006). Principles of two-photon excitation
microscopy and its applications to neuroscience. _Neuron_ 50: 823–839. Article CAS Google Scholar * Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ _et al_ (2013). Long-term
dynamics of CA1 hippocampal place codes. _Nat Neurosci_ 16: 264–266. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Joshua Jennings for assistance in preparing
the figure. We thank the Brain and Behavior Research Foundation, The Foundation of Hope, The Klarman Family Foundation, the National Institute on Drug Abuse (DA032750, DA038168) (GDS), and
the Carolina Institute for Developmental Disabilities (T32HD040127) (SLR) for support. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Departments of Psychiatry & Cell and Molecular
Physiology, UNC Neuroscience Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA Shanna L Resendez & Garret D Stuber * Neuroscience Center, University of North
Carolina at Chapel Hill, Chapel Hill, NC, USA Shanna L Resendez & Garret D Stuber Authors * Shanna L Resendez View author publications You can also search for this author inPubMed Google
Scholar * Garret D Stuber View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Garret D Stuber. POWERPOINT SLIDES
POWERPOINT SLIDE FOR FIG. 1 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Resendez, S., Stuber, G. _In vivo_ Calcium Imaging to Illuminate Neurocircuit
Activity Dynamics Underlying Naturalistic Behavior. _Neuropsychopharmacol_ 40, 238–239 (2015). https://doi.org/10.1038/npp.2014.206 Download citation * Published: 08 December 2014 * Issue
Date: January 2015 * DOI: https://doi.org/10.1038/npp.2014.206 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
