Agonist Medications for the Treatment of Cocaine Use Disorder
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Cocaine addiction is a persistent and insidious public health problem (Pomara et al, 2012). Despite evidence for sustained prevalence, clinical harm, and demand for treatment, the Food and
Drug Administration (FDA) has yet to approve any pharmacotherapy for its treatment. ‘Agonist’ medications such as amphetamine maintenance have emerged as one intriguing but controversial
class of candidates, and this Circumspectives Article presents pros and cons of agonist medications for treatment of cocaine-use disorder. Evidence in favor of agonist medications will be
presented by Dr Steve Negus. Dr Negus has contributed for more than 20 years to research on medications development for drug-abuse treatment, and he published early preclinical data
supporting efficacy of amphetamine maintenance to reduce cocaine self-administration. Regulatory challenges to the use of agonist medications will be presented by Dr Jack Henningfield. Dr
Henningfield also has decades of experience in preclinical and clinical evaluation of both abuse potential of novel drugs and utility of candidate treatments. He was involved in the research
and regulatory processes that led to FDA approval of various nicotine agonist therapies and of buprenorphine as an analgesic and agonist therapy.
Amphetamine maintenance reduces cocaine-taking behavior in rats, nonhuman primates, and humans (Howell and Negus, 2014).
My research on medications development is founded on the premise that consideration of any medication for any indication depends initially on evidence for therapeutic efficacy. Absent that
evidence, little else matters. However, as evidence for efficacy accumulates, then secondary issues related to safety and deployment become increasingly relevant. My advocacy for
consideration of agonist medications to treat cocaine-use disorder stems from the growing body of preclinical and clinical evidence for their therapeutic efficacy.
In considering this evidence, it is useful to begin by offering definitions both for the disease and for metrics of therapeutic efficacy. Substance-use disorders are defined by a maladaptive
allocation of behavior toward acquisition and use of drugs and away from behaviors maintained by alternative reinforcers (Ahmed et al, 2013; American Psychiatric Association, 2013; Banks
and Negus, 2012; Heyman, 2009). From this perspective, treatment efficacy can be defined both by reductions in drug-taking behavior and also by reallocation of behavior toward healthy
alternative activities. Pharmacotherapy can promote this behavioral reallocation by multiple mechanisms (Grabowski et al, 2004; Howell and Negus, 2014; Rothman et al, 2002; Stoops and Rush,
2013). Perhaps the simplest and most intuitive mechanism is an ‘antagonist’ approach that prevents the abused drug from reaching the brain (eg, with vaccines that promote rapid peripheral
metabolism of the abused drug) or from reaching target receptors within the brain (eg, with receptor antagonists). The μ-opioid receptor antagonist naltrexone, which is approved for
treatment of opioid-use disorders, is one example of an antagonist medication that can have high therapeutic efficacy and safety under appropriate conditions. However, compliance has been a
significant obstacle to the use of naltrexone (Comer et al, 2007) and antagonist approaches have not yet succeeded as viable pharmacotherapies for cocaine-use disorder (Gorelick, 2012;
Grabowski et al, 2000; Haney et al, 2001).
‘Agonist’ medications offer a different approach. Agonist medications are drugs that share pharmacodynamic mechanisms of action with the abused drug but that usually have distinct
pharmacokinetic characteristics (eg, enteral bioavailability, slow onset of action, long duration of action) (Grabowski et al, 2004; Rothman et al, 2002). The overlap in pharmacodynamic
mechanisms endows agonist medications with at least three important attributes. First, maintenance on agonist medications can attenuate reinforcing effects of the abused drug by competing
with the abused drug for its receptors and/or by producing cross tolerance to effects of the abused drug at those receptors (Bauer et al, 2014; Kreek et al, 2002). Second, agonist
medications have high probability of functioning as effective reinforcers in patients, and as a result, their delivery in a clinical context can be leveraged to promote compliance and
reinforce other desirable behaviors (Preston et al, 2000). Third, agonist medications can alleviate withdrawal signs that contribute to relapse (Koob, 2009; Negus and Banks, 2013). The
pharmacokinetic attributes of agonist medications enable their use by safer routes of administration (eg, oral and sublingual) than the intravenous or smoked routes common in drug abuse. In
addition, slow drug onset can reduce abuse potential (Lile, 2006) and long duration of action can reduce the frequency of required treatment and also reduce problematic neuroadaptations to
the severe oscillations in drug levels that often occur with drug abuse (Kreek et al, 2012). Of course, agonist medications can also produce undesirable effects similar to those of the
abused drug, and in the context of addiction treatment, abuse liability is an especially prominent concern. Nonetheless, agonist medications have been successfully developed and deployed,
and examples include oral methadone for the treatment of opioid dependence and nicotine formulations for the treatment of tobacco dependence. It was the relative success of these medications
for treatment of addiction to other drugs that stimulated initial research on potential of agonist medications to treat cocaine-use disorder.
Cocaine functions as an inhibitor of dopamine, norepinephrine, and serotonin transporters, and its effects on dopamine are most strongly implicated in its abuse liability (Johanson and
Fischman, 1989; Koob, 1992; Ritz et al, 1987). Consequently, drugs acting as dopamine uptake inhibitors, dopamine releasers, or dopamine receptor agonists have all been evaluated as
candidate agonist medications for treatment of cocaine abuse (Grabowski et al, 2004; Herin et al, 2010; Rothman et al, 2002; Stoops and Rush, 2013). Of these, the most promising effects have
been obtained with dopamine releasers in general, and with amphetamine maintenance in particular. In a series of early preclinical studies, we found that chronic amphetamine treatment
decreased cocaine self-administration in rhesus monkeys responding under several different schedules of cocaine reinforcement, including a ‘choice’ procedure in which monkeys choose between
cocaine injections and food pellets (Figure 1) (Negus, 2003; Negus and Mello, 2003a, 2003b). These initial findings have been replicated and extended by us (Banks et al, 2013b), by other
investigators working with other schedules of cocaine self-administration in nonhuman primates (Czoty et al, 2010; Czoty et al, 2011), and by investigators working with various schedules of
cocaine self-administration in rats (Chiodo et al, 2008; Thomsen et al, 2012). In addition, amphetamine maintenance decreased cocaine vs money choice in human laboratory studies (Greenwald
et al, 2010; Rush et al, 2010), and cocaine use by patients in placebo-controlled double-blind clinical trials (Grabowski et al, 2001; Mariani et al, 2012). Finally, recent meta-analyses of
clinical studies have highlighted agonist medications in general, and amphetamine maintenance in particular, as superior in efficacy to many other drug classes as candidate medications for
treatment of cocaine dependence (Amato et al, 2007; Amato et al, 2011; Castells et al, 2010; Minozzi et al, 2008; Pani et al, 2011a; Pani et al, 2011b).
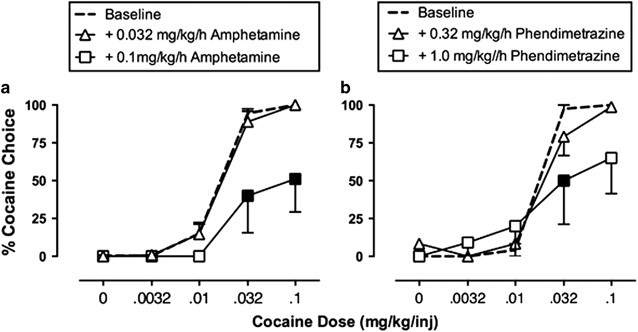
Maintenance on amphetamine (a) or phendimetrazine (b) decreases cocaine vs food choice in rhesus monkeys. During experimental sessions, monkeys (N=4–6) could choose between a single food
pellet and one of a series of increasing cocaine doses. Each graph shows the percentage of cocaine choice on the Y axis as a function of the available cocaine dose on the X axis. Under
baseline conditions, increasing unit doses of self-administered cocaine maintained a dose-dependent increase in cocaine choice. Filled points show significant decreases in cocaine choice
produced by continuous 14-day treatment with intravenous amphetamine or phendimetrazine. Adapted from Banks et al, 2013b.
In an effort to improve both efficacy and safety, more recent research has compared effects of amphetamine with effects of other monoamine releasers, or with other classes of candidate
agonist medications. This work has suggested three general conclusions. First, pharmacological selectivity to release dopamine>serotonin appears to be one determinant of monoamine releaser
efficacy to reduce cocaine choice with minimum side effects (Banks et al, 2011; Haile et al, 2010; Mooney et al, 2009; Negus et al, 2009; Negus et al, 2007; Rothman et al, 2005). Thus,
effects of amphetamine can be mimicked by other dopamine-selective releasers such as phenmetrazine or methamphetamine, but not by serotonin-selective releasers such as fenfluramine. Second,
prodrugs for amphetamine and phenmetrazine can also decrease cocaine choice (Banks et al, 2013a; Banks et al, 2013b). For example, lisdexamfetamine is a Schedule II prodrug for amphetamine
that produces an amphetamine-like reduction in cocaine choice [Banks ML, Hutsell BA, Blough BE, Poklis JL, Negus SS (submitted). Effects of 7-day lisdexamfetamine treatment on choice between
cocaine and food in rhesus monkeys.]. Similarly, phendimetrazine is a prodrug for phenmetrazine that also reduces cocaine choice (Figure 1), and the Schedule III status of phendimetrazine
reduces barriers to its use by physicians. More generally, the efficacy of these prodrugs is relevant insofar as they may have lower abuse liability than their active metabolites. Finally,
studies in both animals and humans have suggested that amphetamine and related monoamine releasers may be safer and/or more efficacious than other classes of candidate agonist medications
including both monoamine uptake inhibitors and dopamine receptor agonists (Czoty et al, 2013; Grabowski et al, 2004; Howell and Negus, 2014). However, the generality and underlying basis for
this apparently higher safety/efficacy of dopamine-selective monoamine releasers has not been explored.
Despite these caveats, maintenance on amphetamine and related monoamine releasers has displayed greater and more reliable therapeutic efficacy to reduce cocaine consumption than any other
type of medication tested to date. Moreover, the translational consistency of amphetamine maintenance to reduce cocaine-taking behaviors across multiple species and experimental procedures
has been striking. In my view, the force of evidence for therapeutic efficacy suggests that secondary issues related to safety and deployment now warrant serious consideration. I would
further submit that reasonable strategies are available (eg, use of prodrugs) to mitigate many of the concerns related to these secondary issues.
In reality, stimulants vary widely in their risks of abuse and risks associated with their use such that the adverse consequences of their use and abuse are far worse for cocaine and
illicitly manufactured amphetamine products as compared with prescribed pharmaceutical stimulants, but this distinction appears lost on many who are involved in drug control as has been
discussed elsewhere (Gerlach et al, 2014). Moreover, as discussed in this commentary, there is no apparent sponsor willing to commit the extensive resources and sustained effort that would
be required to develop the science base sufficiently to enable submission of a New Drug Application (NDA), including development of the Full Prescribing Information, to the FDA and be
prepared to implement the likely requisite postmarketing risk-management program.
Decades of experience with opioid- and nicotine-based agonist therapy provide both supporters and opponents of such therapies an ample arsenal of data and arguments to support their
positions for and against approval of such therapies. From a regulatory perspective, such debates may raise the hurdles for drug approval and slow the review process, because FDA is required
to evaluate all credible safety concerns to provide assurance that the drug products it approves are acceptably safe.
Anticipation of a protracted and burdensome regulatory process can dissuade sponsors and potential investors from initiating and then pursuing such drug development. This is not unique to
drug-dependence treatments or the approval of drugs that are regulated as controlled substances; however, controlled substances require increasingly diverse and complex studies to evaluate
abuse potential, additional documentation in drug application filings (eg, Abuse Potential Assessment), and postmarketing requirements beyond what is typically required of drugs that are not
regulated under the provisions of the Controlled Substances Act (CSA) (Calderon and Klein, 2014; Cone et al, 2013; Dart, 2009; Dasgupta and Schnoll, 2009; FDA, 2009, 2010a, 2013a;
Leiderman, 2009; Schnoll et al, 2006).
Although these charges to FDA may seem straightforward, the reality is that it typically requires many years of effort, many millions of dollars, and many studies to evaluate the specific
drug product that will be submitted for approval. FDA approval of a category of products, eg, stimulants, is not likely. Thus, the labeling for the specific product must include a
scientifically informed basis for dosing recommendations and restrictions intended to assure safe and effective use according to a benefit-risk assessment in the intended population. Studies
may include evaluation of the pharmacokinetics and effects in various subpopulations, and in conjunction with other drugs that are likely to be used in the population, as well as in persons
with co-morbid disease states commonly occurring in the indicated population.
On the supportive side, as summarized by Dr Negus, there has been good progress in developing the scientific foundation supporting the plausibility that stimulant agonist therapy could
ultimately be developed to the point at which science would support an NDA that could be approved as safe and effective. However, all of the work that has been done to date does little more
than test the proof of concept. It appears to be far short of what would likely be necessary to develop a credible NDA as summarized in Table 2. Agonist-based treatment of
cocaine-dependence/use disorder with amphetamine-type stimulants is long on theory and far short of the evidence that would be required for the candidate product that would be submitted in
an NDA. In fact, it is not clear what the specific product entity (‘drug substance’), dosage form, or actual drug product to be submitted for approval would be. Nor is there an apparent
sponsor willing to provide the likely several years of sustained support to develop and evaluate the product and indication for which approval would be sought, to develop and commit to
extensive postmarketing requirements, and to martial a program to garner the broad political support that may be required to facilitate approval and conditions of access that would be
necessary to assure success from both a commercial and public health perspective. Unfortunately, the prospects appear better that fibromyalgia sufferers will have the opportunity to benefit
from sodium oxybate therapy.
A substantial and growing body of evidence supports the potential therapeutic effectiveness of amphetamine and pharmacologically related ‘agonist’ medications for treatment of cocaine-use
disorder, but clear obstacles exist to the acceptance and deployment of such medications. In this concluding section, the authors consider two paths for future research and development that
might advance this field. A third issue raised during peer review of the manuscript will also be briefly addressed.
Although existing research provides strong evidence for effectiveness of amphetamine maintenance to reduce cocaine consumption, several outstanding questions remain, and research on these
questions could contribute both to the development of improved medication candidates and to better understanding of basic processes in drug abuse. The authors of this circumspectives article
agreed that two of those questions warrant discussion here. First, we have little information on the degree to which amphetamine-like medications that reduce cocaine consumption might also
reduce consumption of other abused stimulants. Cocaine inhibits dopamine transporters, and some other drugs of abuse, such as methylenedioxypyrovalerone (MDPV), share this mechanism of
action (Baumann et al, 2012b; Cameron et al, 2013). However, MDPV is both more potent and more selective than cocaine as a dopamine transporter inhibitor, and it is unknown whether
amphetamine maintenance regimens that reduce cocaine consumption might also reduce consumption of MDPV or other abused dopamine transporter inhibitors. Notably, some other treatments have
been found to differentially reduce abuse-related effects of cocaine vs MDPV (Bonano et al, 2014). The general class of abused stimulants also includes drugs such as amphetamine,
methamphetamine, and emerging cathinone analogs that function as substrates at dopamine transporters to promote dopamine release (Baumann et al, 2012a; Cameron et al, 2013; Rothman et al,
2001). Recent clinical trials and a human laboratory study found that amphetamine maintenance failed to produce significant decreases in methamphetamine consumption (Galloway et al, 2011;
Longo et al, 2010; Pike et al, 2014). These and related results have been interpreted to suggest that dopamine releasers such as amphetamine may be more effective to treat abuse of dopamine
uptake inhibitors (eg, cocaine) than abuse of other dopamine releasers.
A second question that warrants further research is the mechanism that underlies amphetamine-induced decreases in cocaine use. One early hypothesis was that amphetamine maintenance might
produce pharmacodynamic tolerance to its own effects and cross tolerance to the abuse-related effects of cocaine, eg, by downregulating dopamine transporters (Fleckenstein et al, 1999; Negus
and Mello, 2003b). However, recent evidence does not support this hypothesis, and suggests instead that amphetamine maintenance may increase basal tone in dopaminergic signaling and thereby
reduce abuse-related changes in dopamine signaling produced by cocaine (Bauer et al, 2014). A related question is whether mechanisms of the anti-cocaine effects of amphetamine maintenance
can be dissociated from mechanisms of its side effects in general and its abuse-related effects in particular. In support of this possibility, a large body of research suggests that a subset
of amphetamine effects, including its abuse-related effects, is mediated in part by increases in endogenous opioid release and activation of opioid receptors. For example, the opioid
antagonists naloxone and/or naltrexone blunted abuse-related neurochemical and behavioral effects of amphetamine in microdialysis assays of dopamine release in the striatum (Hitzemann et al,
1982; Hooks et al, 1992) and in behavioral assays of intracranial self-stimulation in rats (Esposito et al, 1980; Holtzman, 1976), place conditioning in rats (Trujillo et al, 1991), and
amphetamine self-administration in rhesus monkeys (Jimenez-Gomez et al, 2011). Naltrexone also reduced amphetamine subjective effects in non-dependent and amphetamine-dependent subjects, and
reduced amphetamine abuse in a placebo-controlled double-blind clinical trial (Jayaram-Lindstrom et al, 2008a; Jayaram-Lindstrom et al, 2008b; Jayaram-Lindstrom et al, 2004). However,
opioid antagonists do not block all amphetamine effects (van Kammen and Schulz, 1985; Winslow and Miczek, 1988; Wiskerke et al, 2011; Woolfolk and Holtzman, 1997), and it is unknown whether
opioid mechanisms contribute to the anti-cocaine effects of amphetamine. Overall, research on these or other possible amphetamine mechanisms may suggest new strategies to dissociate the
anti-cocaine effects of amphetamine from its undesirable effects.
Current preclinical and clinical trials of candidate medications for substance-use disorders assess medication efficacy by focusing on measures of drug-taking behavior and drug consumption
(eg, rate of drug self-administration in animal studies and number of cocaine-positive urines in clinical trials). The FDA has also focused on measures of drug use as primary outcome
measures in reviews of medications advanced as candidates to treat abuse of opioids, tobacco, and alcohol, as well as cocaine. For drugs other than tobacco, duration of participation in
treatment (‘treatment retention’) is an additional important efficacy measure, and a variety of other measures may also be used in clinical trials and/or follow-up studies to assess
potential treatment benefits, eg, criminal activity, job retention, and development of diseases such as HIV AIDS. These other measures may be important in justifying the benefits of
treatment to payers and society at large, but they are not considered primary efficacy outcome measures on which to base drug approval. During peer review of this manuscript, it was noted
that metrics of drug use are surrogate measures for clinically relevant outcomes that also include morbidity and mortality, and acceptance of agonist medications (or any other type of
medication) for treatment of cocaine-use disorder will ultimately depend on evidence for reductions not only in cocaine use, but also in morbidity and mortality associated with cocaine use.
The authors of this circumspectives article agree that morbidity and mortality are important concerns in treatment of drug abuse, and we agree that effective treatments should ultimately
reduce their incidence. However, we do not recommend their inclusion as clinical endpoints for FDA approval for three reasons. First, the approval of all existing medications for
substance-use disorders has been based primarily on efficacy to reduce measures of drug use, and such measures have proved valid as predictors of treatment outcome in clinical practice.
Thus, it is our view that drug-use metrics have served as appropriate, accessible, and quantitative measures for evaluation of other drug-abuse treatments, and these criteria should also be
appropriate for review of candidate medications for treatment of cocaine-use disorder. Second, the taxonomy of morbidity and mortality outcomes in cocaine-use disorder has not been clearly
delineated or prioritized, and methods for quantifying those outcomes and incorporating them into preclinical and clinical research designs have not been adequately defined. This may be a
topic for future research. Finally, and related to the second point, mortality is an impractical measure in medication assessment, because its incidence is relatively low, and as a result,
very large numbers of patients would need to be retained in treatment for long periods at a great expense to collect data sufficient for statistical analysis. We believe that any commercial
sponsor would be
unlikely to commit the resources and accept the high risks of uncertain outcome using such measures. Nonetheless, we agree that just as epidemiological studies have been useful in
documenting the public health benefits of treatment for other substance-abuse disorders, so too should such studies be conducted to assess consequences of agonist therapies for cocaine
abuse.
Dr Negus declares that his research has been funded by grants from the National Institutes of Health, including R01DA026946. During the past 3 years, he has received compensation as a
consultant for or collaborator with the pharmaceutical companies Alkermes and Grunenthal for projects related to opioid pharmacology and assessment of abuse liability. Dr Negus declares that
the present study was not related to this professional relationship and should not be perceived as constituting a potential conflict of interest. Dr Henningfield provides consulting
services through PinneyAssociates to pharmaceutical companies to support the development, approval, and appropriate regulation of medications for a broad variety of diseases including opioid
and nicotine medications for treating opioid and tobacco dependence respectively, and the use of stimulants for treating attention deficit hyperactivity disorder and other diseases. This
work includes abuse potential assessment, risk management, post-marketing surveillance, drug scheduling, and other regulatory requirements for CNS acting drugs with a potential for abuse and
dependence. Through Pinney Associates, this consulting has included GlaxoSmithKline’s nicotine replacement products for tobacco dependence treatment, and Shire’s stimulant medications for
treatment of ADHD. Dr Henningfield’s time and effort on this manuscript were supported by PinneyAssociates without support for or input from any such commercial interests.
Anyone you share the following link with will be able to read this content:
