Effects of intranasal oxytocin on the blood oxygenation level-dependent signal in food motivation and cognitive control pathways in overweight and obese men
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Recent research indicates that the hypothalamic neuropeptide hormone oxytocin is a key central nervous system factor in the regulation of food intake and weight. However, the
mechanisms underlying the anorexigenic effects of oxytocin in humans are unknown and critical to study to consider oxytocin as a neurohormonal weight loss treatment. We performed a
randomized, double-blind, placebo-controlled crossover study with single-dose intranasal oxytocin (24 IU) in ten overweight or obese, otherwise healthy men. Following oxytocin/placebo
administration, participants completed an established functional magnetic resonance imaging food motivation paradigm. We hypothesized that oxytocin would reduce the blood oxygenation
level-dependent (BOLD) signal to high-calorie food _vs_ non-food visual stimuli in the ventral tegmental area (VTA), the origin of the mesolimbic dopaminergic reward system. Following
oxytocin administration, compared to placebo, participants showed bilateral VTA hypoactivation to high-calorie food stimuli. A secondary exploratory whole-brain analysis revealed
hypoactivation in additional hedonic (orbitofrontal cortex, insula, globus pallidus, putamen, hippocampus, and amygdala) and homeostatic (hypothalamus) food motivation and hyperactivation in
cognitive control (anterior cingulate and frontopolar cortex) brain regions following oxytocin administration _vs_ placebo. Oxytocin administration reduces the BOLD signal in reward-related
food motivation brain regions, providing a potential neurobiological mechanism for the anorexigenic oxytocin effects in humans. Furthermore, our data indicate that oxytocin administration
reduces activation in homeostatic and increases activation in cognitive control brain regions critically involved in regulating food intake and resolving affective conflict, respectively.
Future studies are required to link these changes in brain activation to oxytocin effects on food intake and weight. SIMILAR CONTENT BEING VIEWED BY OTHERS SEX DIFFERENCES IN CENTRAL INSULIN
ACTION: EFFECT OF INTRANASAL INSULIN ON NEURAL FOOD CUE REACTIVITY IN ADULTS WITH NORMAL WEIGHT AND OVERWEIGHT Article Open access 17 June 2022 ACTS OF APPETITE: NEURAL CIRCUITS GOVERNING
THE APPETITIVE, CONSUMMATORY, AND TERMINATING PHASES OF FEEDING Article 25 July 2022 HOMEOSTASIS AND FOOD CRAVING IN OBESITY: A FUNCTIONAL MRI STUDY Article Open access 17 August 2021
INTRODUCTION Oxytocin is currently under investigation as a potential novel neurohormonal treatment for obesity (Blevins and Baskin, 2015). Obesity represents a major public health concern
in need of more effective and tolerable treatments. In the United States, 69% of adults are overweight with 35% meeting the criteria for obesity, an epidemic that is associated with diabetes
mellitus and increased mortality (Ogden et al, 2014). Even though complications resulting from obesity may be reversible with weight reduction, meaningful weight loss is difficult to
achieve and maintain with available medical treatments. In addition, current treatment options are associated with frequent complications (bariatric surgery; Gloy et al, 2013) and
significant side effects (FDA-approved drugs; Yanovski and Yanovski, 2014). The nonapeptide hormone oxytocin is primarily produced in the paraventricular and supraoptic nuclei of the
hypothalamus and secreted throughout the brain and to the peripheral circulation via the posterior pituitary. Repeated oxytocin administration has been shown to induce significant weight
loss in rodent, primate, and human obesity (Blevins and Baskin, 2015; Blevins et al, 2015; Zhang et al, 2013). Importantly, short-term administration in humans has been associated with
minimal side effects (Anagnostou et al, 2012; Zhang et al, 2013). In animal models, the beneficial effects of oxytocin on weight are due to multiple processes, including increased energy
expenditure and lipolysis and reduced food intake (Blevins and Baskin, 2015). For example, in fasting rats, central and peripheral oxytocin administration resulted in a dose-dependent
reduction in caloric intake and an increased delay before eating (Arletti et al, 1989; Olson et al, 1991). This effect was reversed by administering an oxytocin antagonist prior to oxytocin
exposure, suggesting that this effect is mediated by oxytocin receptors (Arletti et al, 1989). Recent translational studies using intranasal oxytocin have demonstrated that oxytocin reduces
caloric consumption, particularly of palatable foods, without affecting subjective appetite in men (Lawson et al, 2015; Ott et al, 2013; Thienel et al, 2016). However, the mechanisms driving
the effect of oxytocin on food intake in humans are unknown, and an understanding of these mechanisms is critical for the development of oxytocin-based therapies for obesity management.
Preclinical data indicate that oxytocin may reduce food intake in part by modulating activation of hedonic food motivation pathways. Although homeostatic modulation of food intake
determining hunger and appetite is largely controlled by the hypothalamus, which receives and integrates peripheral anorexigenic and orexigenic signals, hedonic food motivation arises from a
widespread network of brain regions known to be involved in reward processing. The ventral tegmental area (VTA), amygdala, insula, basal ganglia (eg, nucleus accumbens), hippocampus, and
orbitofrontal cortex (OFC) are associated with hedonic regulation of human appetite, as shown by their selective functional magnetic resonance imaging (fMRI) activation to palatable food
stimuli (Holsen et al, 2012; LaBar et al, 2001). At the core of this mesolimbic dopaminergic reward system, the VTA is the origin of its dopaminergic cell bodies and features oxytocin
receptors (Loup et al, 1991). In addition, oxytocin neurons project from the paraventricular and supraoptic nuclei of the hypothalamus to the VTA (Sofroniew, 1983). In preclinical studies,
oxytocin availability in the VTA has been shown to alter dopamine levels in brain regions downstream from the VTA (eg, in the nucleus accumbens; Melis et al, 2007), providing a potential
biological link between oxytocin and motivational behavior including hedonic food intake. In rats, administration of oxytocin into the VTA and nucleus accumbens reduced sucrose intake,
whereas these effects were attenuated when an oxytocin receptor blocker was given prior to oxytocin treatment (Herisson et al, 2016; Mullis et al, 2013). In humans, obesity is characterized
by fMRI hyperactivation of food motivation brain regions associated with hedonic aspects of appetite and food intake (De Silva et al, 2012; Dimitropoulos et al, 2012). Although human studies
provide evidence that single-dose intranasal oxytocin administration alters the blood oxygenation level-dependent (BOLD) signal to social and emotional stimuli (Domes et al, 2007; Wigton et
al, 2015) and monetary rewards (Mickey et al, 2016; Nawijn et al, 2016), to our knowledge, the impact of oxytocin on the spontaneous BOLD signal in reward-related food motivation pathways
in response to viewing palatable food images has not yet been examined. We therefore performed a randomized, double-blind, placebo-controlled crossover pilot study of 24 IU intranasal
oxytocin in overweight and obese, otherwise healthy men to investigate oxytocin effects on the BOLD signal in hedonic food motivation brain regions using a well-established visual food
stimuli fMRI paradigm (Holsen et al, 2012). We hypothesized that when participants view high-calorie foods compared to objects, the BOLD signal in the VTA as a key node for hedonic food
motivation would be reduced following oxytocin administration compared to placebo. As the hedonic regulation of appetite emerges from a network of food motivation regions extending beyond
the VTA and given that other non-reward functions (eg, homeostasis, cognitive control; Calvo et al, 2014) and their according brain regions (eg, hypothalamus for homeostasis and dorsolateral
prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), and frontopolar cortex for cognitive control; Niendam et al, 2012; Saper et al, 2002) are likely to be involved in determining
food intake, we performed whole-brain fMRI and, as a secondary exploratory aim, examined the effects of oxytocin on the BOLD signal across the entire brain. MATERIALS AND METHODS
PARTICIPANTS Ten overweight or obese men, recruited from the community, took part in the study. Inclusion criteria were an age between 18 and 45 years, a body mass index (BMI) between 25 and
40 kg/m2, regular breakfast eating (⩾4 times per week), and stable weight over the past 3 months. Exclusion criteria were a psychiatric diagnosis, psychotropic medication, a history of
eating disorder by Structured Clinical Interview for DSM Disorders—IV (SCID-IV), excessive exercise (running >25 miles or exercising >10 h in any 1 week) within the last 3 months,
active substance abuse, smoking, a history of cardiovascular disease, diabetes mellitus, or gastrointestinal tract surgery, hematocrit below the normal range, untreated thyroid disease, and
contraindication for MRI. PROCEDURES This study was approved by the Partners HealthCare Institutional Review Board and conducted in accordance with the Declaration of Helsinki. Following the
International Committee of Medical Journal Editors’ registration requirement, this clinical trial was preregistered at clinicaltrials.gov (registration number: NCT02276677). All
participants provided their written informed consent prior to participation. Participants were admitted to the Massachusetts General Hospital Clinical Research Center for outpatient
screening and two main study visits. At screening, medical history, physical examination (including height and weight measurements), and a blood draw were performed to confirm eligibility.
Exercise pattern was assessed by medical history and Paffenbarger Physical Activity Scale (Paffenbarger et al, 1993). In addition, behavior related to food intake was assessed with the
Three-Factor Eating Questionnaire (TFEQ), a 51-item self-report questionnaire that provides scores for three factors determining eating behavior, ie, cognitive restraint of eating,
disinhibition, and hunger (Stunkard and Messick, 1985). The first main study visit was completed between 6 and 49 days after the screening visit. Screening visits were conducted between
November 2014 and May 2015. Main study visits were completed between December 2014 and May 2015. Main study visits took place in the morning between 6 and 24 days apart. Participants
completed a food diary for the 72 h leading up to each main study visit. In addition, they were asked to fast for at least 10 h and avoid strenuous exercise and alcohol consumption in the 24
h prior to study visits. Each main study visit started with an update of the medical history and collection of the completed food diary. At visit 1, a copy of the food diary from the 72 h
leading up to the visit was provided to the participant who was instructed to replicate the recorded eating pattern as closely as possible prior to presenting for visit 2. From the food
diaries, a dietician analyzed the nutritional content of the food intake (total caloric intake as well as carbohydrate, fat, and protein content) in the 72 h prior to both main study visits
for each participant using ProNutra (Viocare, Princeton, NJ, USA) to confirm similar conditions preceding oxytocin and placebo visits. In addition, self-reported duration of sleep during the
night prior to each main study visit was documented. At 0730 hours, intranasal oxytocin (24 IU, Syntocinon, Novartis, Switzerland, provided by Victoria Pharmacy Zürich, Switzerland) or
placebo (same inactive ingredients and packaging, Victoria Pharmacy) nasal spray (5 ml bottles with 40 IU/ml oxytocin or placebo and a 0.1 ml dosage pump) was self-administered with three
sprays per nostril under the supervision of a nurse practitioner. For this randomized, double-blind, placebo-controlled crossover study, the research pharmacy randomized the participants 1:1
to one of two drug orders, ie, oxytocin—placebo or placebo—oxytocin. All study personnel and participants were blinded to the randomization. Sixty minutes after oxytocin or placebo
administration, participants underwent fMRI. Immediately prior to as well as 30 and 90 min following oxytocin/placebo administration, appetite was assessed on the dimensions Hunger and
Desire to Eat Favorite Food on visual analog scales of 10-cm length, anchored by ‘not at all’ on the left and ‘extremely’ on the right. At the same time points, current levels of anxiety
were measured using the state scale of the State-Trait Anxiety Inventory (STAI; Spielberger et al, 1983). For 20 statements (eg, ‘I feel nervous.’), participants were asked to indicate how
much the statement matched their current feelings on a four-point Likert-like scale ranging from 1 (‘not at all’) to 4 (‘very much so’). FUNCTIONAL MRI PARADIGM Functional MRI scanning was
performed during a well-established food motivation paradigm that has been reported in detail elsewhere (Holsen et al, 2012). In short, participants viewed 100 high-calorie food stimuli (50
savory, 50 sweet items), 100 low-calorie food stimuli, 100 household objects, and 100 fixation stimuli in a block design. Each stimulus was presented once for 3 s using Presentation software
(Neurobehavioral Systems, Albany, CA, USA). Participants were instructed to press a button when pictures changed to insure their attention to stimuli. A total of five 4-min runs with five
images in each block and 16 blocks in each run were completed. MRI ACQUISITION PARAMETERS MRI data were acquired using a Siemens 3T Trio scanner (Siemens, Erlangen, Germany) at the Athinoula
A. Martinos Center for Biomedical Imaging. Head movements were restricted with foam cushions. Whole-brain functional imaging was performed using a gradient-echo EPI pulse sequence (33
contiguous oblique-axial slices, 4-mm thick, TR/TE=2000/30 ms, flip angle=90°, FOV=200 × 200 mm, 120 total images per run). DATA ANALYSIS Comparisons of food intake, sleep duration,
appetite, and anxiety between oxytocin and placebo visits were analyzed with Wilcoxon signed-rank tests using JMP Pro (version 13; SAS Institute, Cary, NC, USA). Functional MRI data were
processed using Statistical Parametric Mapping 8 (SPM8; Wellcome Department of Cognitive Neurology, London, UK; www.fil.ion.ucl.ac.uk/spm). Functional MRI images were slice-time corrected,
realigned, spatially normalized, and registered to the Montreal Neurological Institute (MNI; www.bic.mni.mcgill.ca) T1 canonical template. Data were then resampled to 2 mm3 voxels and
smoothed with a 6-mm isotropic full width at half-maximum Gaussian function. All collected data showed minimal head motion (<3 mm linear movement in the orthogonal planes and <0.5°
radians of angular movement). For each participant, condition effects were estimated at each voxel, and statistical parametric maps (con images) were produced for the high-calorie food and
non-food object conditions for both the oxytocin and placebo conditions. As the primary region of interest (ie, VTA) is very small, preprocessing of oxytocin and placebo conditions was done
within the same pipeline to ensure proper alignment of individual scans. These individual con images were then entered into a second-level analysis using a linear flexible factorial model
with the factors participant and condition (oxytocin high-calorie stimuli, oxytocin non-food objects, placebo high-calorie stimuli, and placebo non-food objects). Oxytocin-related changes
were tested using contrasts that reflected the difference in beta weights between oxytocin high-calorie food images minus oxytocin non-food objects _vs_ placebo high-calorie food images
minus placebo non-food objects. For the primary confirmatory analysis of oxytocin effects on VTA activation, we used max voxel coordinates from the left and right hemispheres of the group
level statistical maps at the centers of 2.5 mm spheres and the beta values using Marsbar software. The location of the VTA was verified by a senior neuroanatomist (NM) guided by MRI-visible
anatomical landmarks and brain atlases (Parent, 1996; Paxinos and Huang, 1995). Following the primary analysis, we conducted an exploratory whole-brain analysis (oxytocin high-calorie food
images minus oxytocin non-food objects _vs_ placebo high-calorie food images minus placebo non-food objects) with a group level statistical map threshold of _p_<0.05 (uncorrected) with
the additional requirement that at least four contiguous voxels exceeded this statistical level. Cohen’s _d_ is reported as effect size measure calculated from the mean _t_-statistic for
each cluster. RESULTS PARTICIPANT CHARACTERISTICS Participant characteristics at the screening visit are described in Table 1. Participants were between 23 and 43 years old, and their BMI
ranged from 25.3 to 33.7 kg/m2. They reported minimal to moderate cognitive restraint of eating, disinhibition, and hunger in their everyday life as indicated by the TFEQ (Table 1). As
intended per study design, food intake (ie, total caloric intake and macronutrient content) during the 72 h leading up to the visits did not differ between oxytocin and placebo visits (Table
2). There were also no differences in sleep duration leading up to the visits. Furthermore, no differences in appetite or anxiety levels were observed between oxytocin and placebo visits
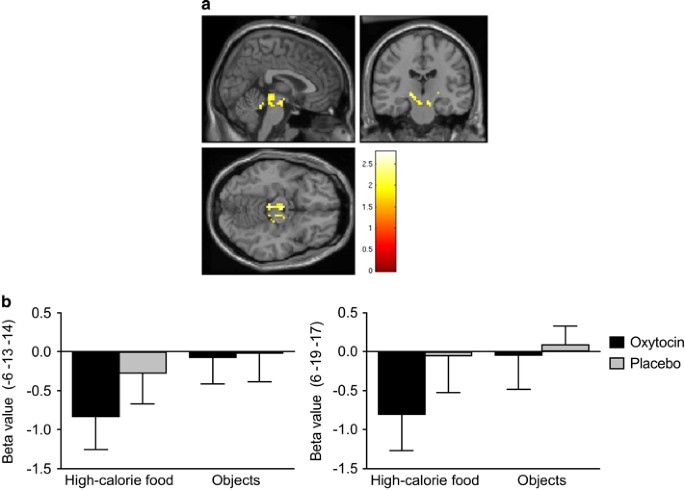
(Table 2). VTA ACTIVATION FOLLOWING OXYTOCIN ADMINISTRATION _VS_ PLACEBO Following oxytocin administration, compared to placebo, participants showed hypoactivation in the left VTA (−6 −13
−14) in response to viewing high-calorie food stimuli compared with the objects, _p_=0.004, _d_=0.91. We observed a matching hypoactivation in the right VTA (6 −19 −17), _p_=0.015, _d_=0.73
(Figure 1). WHOLE-BRAIN ANALYSIS FOLLOWING OXYTOCIN ADMINISTRATION _VS_ PLACEBO Whole-brain analysis contrasting high-calorie food stimuli with non-food objects showed that oxytocin reduced
fMRI activation in additional reward-related food motivation brain regions, including the medial frontal gyrus (Broadmann areas (BAs) 8–11, 45, and 46), precentral gyrus (BA 6), sulcus
callosomarginalis, posterior cingulate gyrus (BA 31), insula, globus pallidus, caudal putamen, thalamus, hippocampus, and amygdala (Table 3). A particularly large hypoactivation following
oxytocin administration _vs_ placebo was observed in BAs 9/45 of the medial frontal gyrus, comprising a cluster of activation of 1551 voxels at the _p_<0.05 (uncorrected) threshold. In
addition, oxytocin (_vs_ placebo) reduced activation in the hypothalamus, a brain area central to homeostatic control of feeding. In contrast, oxytocin increased fMRI activation in brain
regions involved in cognitive control, ie, the superior frontal gyrus (BA 10) and ACC (BA 32; Table 3). DISCUSSION Although translational studies with intranasal oxytocin have recently
demonstrated that oxytocin reduces food intake in men across the weight spectrum (Lawson et al, 2015; Ott et al, 2013; Thienel et al, 2016), the mechanisms driving these anorexigenic effects
of oxytocin in humans are unknown. We tested the impact of single-dose intranasal oxytocin (24 IU) on the BOLD signal in hedonic food motivation pathways while participants viewed
high-calorie food _vs_ non-food stimuli. We hypothesized that the BOLD signal in the VTA as the origin of the mesolimbic dopaminergic reward system would be reduced after oxytocin
administration compared to placebo. Confirming our hypothesis, we found that following oxytocin administration, overweight and obese men showed bilateral hypoactivation in the VTA when
viewing high-calorie food stimuli compared to objects. To our knowledge, this is the first report of reduced VTA activation to palatable food stimuli after oxytocin administration. It is
possible that the attenuating effect of oxytocin on the VTA BOLD signal to rewarding stimuli might be of a more general nature. Two studies have previously shown evidence for oxytocin
effects on the VTA BOLD signal in food-unrelated contexts and populations different from the overweight and obese men investigated in this study. Gregory et al (2015) showed an increased VTA
BOLD response to both crying infant and sexual stimuli in postpartum and nulliparous women after a single dose of 24 IU intranasal oxytocin (the same dose as used in this study,
investigating VTA responses to arousing rather than reward stimuli). Mickey et al (2016) showed an altered time course of VTA activation to monetary reward stimuli following intranasal
oxytocin administration compared to placebo in healthy men. These findings together with our result of VTA hypoactivation to high-calorie food stimuli following oxytocin raise novel
questions of oxytocin use in conditions characterized by altered reward sensitivity (eg, substance abuse). In the context of human obesity, presenting high-calorie food stimuli induces
hyperactivation of hedonic food motivation pathways as compared to normal-weight controls (De Silva et al, 2012; Dimitropoulos et al, 2012). A recent systematic review provides evidence that
obese individuals show increased reward sensitivity not only to food but also to monetary rewards (Stojek and MacKillop, 2017). This points toward a generally increased reward sensitivity
in this population, and the attenuating effect of oxytocin on the VTA BOLD signal might thus represent a promising counteracting intervention. A secondary exploratory analysis assessing
oxytocin effects on the BOLD signal across the entire brain complemented the finding of VTA hypoactivation in response to high-calorie stimuli following oxytocin administration with
hypoactivation of other key brain areas involved in hedonic food motivation. In addition to the VTA, hedonic food motivation pathways comprise the OFC, insula, parts of the basal ganglia,
hippocampus, and amygdala (Holsen et al, 2012; LaBar et al, 2001). In response to oxytocin, we found hypoactivation in the medial frontal gyrus including the OFC (BA 11), insula, basal
ganglia (globus pallidus, putamen), hippocampus, and amygdala, suggestive of widespread oxytocin effects on the hedonic food motivation neurocircuitry. Furthermore, the whole-brain analysis
revealed hypoactivation in the hypothalamus, the core brain region associated with homeostatic food motivation (Saper et al, 2002), following oxytocin compared to placebo. This is consistent
with preclinical data showing that the human ventromedial hypothalamus, representing a brain region that is linked to the control of food intake, is rich in oxytocin receptors (Boccia et
al, 2013; Loup et al, 1991). Moreover, a recent study in healthy, normal-weight individuals showed a reduction of hypothalamic activation to visual food stimuli following a single-dose
administration of 24 IU intranasal oxytocin (van der Klaauw et al, 2017). Our study extends this finding by providing first evidence that intranasal oxytocin similarly impacts hypothalamus
activation in overweight and obese men, where it could serve as a potential weight loss treatment. These data suggest that oxytocin effects on food motivation might not be limited to hedonic
food motivation but also extend to homeostatic food regulation. Investigating the contribution of both food motivation pathways to the anorexigenic effects of oxytocin represents an
interesting scope for future research. Finally, we observed effects of oxytocin on regions involved in cognitive control, the processes that enable individuals to suppress behavioral
impulses to achieve an internal goal (Miller and Cohen, 2001). The ability to exert control over behavior emerges from a complex neural network centered around the DLPFC (BAs 9 and 46), ACC
(BA 32), and frontopolar cortex (BA 10) with connections to other cortical and subcortical (eg, putamen, globus pallidus) brain regions (Niendam et al, 2012). Our exploratory analysis
revealed increased activation in the ACC (BA 32) and frontopolar cortex (BA 10)—brain regions that can be linked to cognitive control—following oxytocin administration compared to placebo,
whereas no other hyperactivations were observed across the entire brain. Cognitive control is assumed to mediate food intake in overweight and obese individuals, as overweight and obese
individuals show reduced cognitive control compared with the normal-weight individuals (Nederkoorn et al, 2006; Sellaro and Colzato, 2017), and lower cognitive control has been associated
with higher levels of uncontrolled eating and increased food intake in this population (eg, Calvo et al, 2014). Oxytocin receptors are evident in key cognitive control regions (Skuse and
Gallagher, 2009), and oxytocin administration has been demonstrated to promote cortical control over subcortical brain structures by increasing connectivity between neocortical and limbic
structures and decreasing limbic-midbrain communication (Domes et al, 2007; Riem et al, 2012). Indeed, a recent study in healthy women showed a trend for an effect of oxytocin in reducing
food craving when instructions of actively suppressing food cravings were given (Striepens et al, 2016). This finding was accompanied by an increased BOLD signal in cognitive control brain
areas following administration of 24 IU intranasal oxytocin compared to placebo. Our study shows evidence of an oxytocin-cognitive control link in overweight and obese individuals.
Furthermore, our study fundamentally differs from Striepens et al (2016) by demonstrating that oxytocin increased activation in cognitive control brain regions even when no suppression of
food cravings was required (as no actual food was present) or requested (eg, via instructions to suppress arising food cravings). We speculate that activation of cognitive control brain
regions in our paradigm results from a priming (ie, automatic activation) of cognitive control by images of palatable foods. For overweight and obese individuals, palatable food stimuli have
a high reward value, driving increased intake of those foods (De Silva et al, 2012; Dimitropoulos et al, 2012). At the same time, (over-)consumption of high-calorie food contributes to
maintaining and increasing overweight in those individuals. Thus, in this population, palatable foods represent stimuli with conflicting connotation (approach and avoidance) and action
implications attached to them. To abstain from (over-)eating available palatable foods, the desire for consuming them may require counteraction with an increase in cognitive control.
Repeated experience of this scenario might eventually result in a well-learned link between palatable foods as aversive signals and increased control demands. This would aid establishing an
automatic activation of the cognitive control network in response to palatable food stimuli (Botvinick, 2007; Dreisbach and Fischer, 2012), and oxytocin appears to amplify this automatic
recruitment of the cognitive control neurocircuitry. This idea is also supported by the fact that the BOLD signal in the ACC (BA 32), an area that detects and signals the need for cognitive
control (Mansouri et al, 2009), is amplified following oxytocin administration _vs_ placebo. More specifically, the area of hyperactivation within the (rostral) ACC following oxytocin
administration _vs_ placebo (−15 44 −5) in this crossover study has been implicated in the detection and resolution of affective conflict (Whalen et al, 1998). Future research is required to
investigate the potential role of this hyperactivation in cognitive control brain regions in the observed hypoactivation in food motivation pathways following oxytocin administration
compared to placebo. This is the first pilot study investigating the effects of oxytocin on the BOLD signal in response to high-calorie food images in overweight and obese men, and the small
sample size represents a limitation of this investigation. Future studies are required to replicate our finding of bilateral VTA hypoactivation to palatable food _vs_ non-food images
following oxytocin administration with larger sample sizes. This study focused on men due to the fluctuations in levels of estrogen and progesterone, hormones that regulate oxytocin
secretion and receptor distribution (Maldjian et al, 2003), in women. However, it will be important to also study women in the future due to gender specificity of oxytocin effects (Gao et
al, 2016). The results of the secondary exploratory whole-brain analysis allow for generating clear hypotheses for future confirmatory studies of oxytocin effects on food motivation and
cognitive control pathways in human obesity. Those investigations should also test whether the observed changes of the BOLD signal in food motivation and cognitive control brain regions are
accompanied by meaningful behavioral changes. Some brain regions, such as the putamen and insula, have a role in both reward processing and cognitive control. Thus, future studies are needed
to understand the functionality of the observed activity changes, for example, by analyzing fMRI during instructed engagement or disengagement of cognitive control and by using functional
connectivity analysis approaches. It can be summarized that key regions of cognitive control are hyperactivated following oxytocin compared to placebo in overweight and obese men, whereas
regions of hedonic and homeostatic food motivation are more robustly hypoactivated. Alterations in hedonic food motivation and cognitive control are likely to both be critically involved in
the anorexigenic effects of oxytocin, as it has been shown that cognitive control and food reward sensitivity combined (with food reward sensitivity measured with the Power of Food Scale and
cognitive control assessed by the ability to choose larger, delayed over smaller, immediate rewards) represent the best predictor of palatable food intake across the weight spectrum
(Appelhans et al, 2011; Ely et al, 2015). FUNDING AND DISCLOSURE This study was supported by the Boston Nutrition Obesity Research Center/NIH Grant 5P30DK046200-20, Nutrition Obesity
Research Center at Harvard/NIH Grant P30-DK040561, and NIH Grant K23-MH092560. Dr Lawson is a member of the scientific advisory board of OXT Therapeutics and has a financial interest in the
company. This company was not involved in any way in this research. The remaining authors declare no conflict of interest. REFERENCES * Anagnostou E, Soorya L, Chaplin W, Bartz J, Halpern D,
Wasserman S _et al_ (2012). Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. _Mol Autism_ 3: 16. Article CAS
Google Scholar * Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R (2011). Inhibiting food reward: delay discounting, food reward sensitivity, and palatable food intake
in overweight and obese women. _Obesity_ 19: 2175–2182. Article Google Scholar * Arletti R, Benelli A, Bertolini A (1989). Influence of oxytocin on feeding behavior in the rat. _Peptides_
10: 89–93. Article CAS Google Scholar * Blevins JE, Baskin DG (2015). Translational and therapeutic potential of oxytocin as an anti-obesity strategy: insights from rodents, nonhuman
primates and humans. _Physiol Behav_ 152 (Pt B): 438–449. Article CAS Google Scholar * Blevins JE, Graham JL, Morton GJ, Bales KL, Schwartz MW, Baskin DG _et al_ (2015). Chronic oxytocin
administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. _Am J Physiol Regul Integr Comp Physiol_ 308: R431–R438.
Article CAS Google Scholar * Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA (2013). Immunohistochemical localization of oxytocin receptors in human brain. _Neuroscience_ 253:
155–164. Article CAS Google Scholar * Botvinick MM (2007). Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. _Cogn Affect Behav
Neurosci_ 7: 356–366. Article Google Scholar * Calvo D, Galioto R, Gunstad J, Spitznagel MB (2014). Uncontrolled eating is associated with reduced executive functioning. _Clin Obes_ 4:
172–179. Article CAS Google Scholar * De Silva A, Salem V, Matthews PM, Dhillo WS (2012). The use of functional MRI to study appetite control in the CNS. _Exp Diabet Res_ 2012: 764017.
Google Scholar * Dimitropoulos A, Tkach J, Ho A, Kennedy J (2012). Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. _Appetite_ 58:
303–312. Article Google Scholar * Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC (2007). Oxytocin attenuates amygdala responses to emotional faces regardless of valence.
_Biol Psychiatry_ 62: 1187–1190. Article CAS Google Scholar * Dreisbach G, Fischer R (2012). Conflicts as aversive signals. _Brain Cogn_ 78: 94–98. Article Google Scholar * Ely AV,
Howard J, Lowe MR (2015). Delayed discounting and hedonic hunger in the prediction of lab-based eating behavior. _Eat Behav_ 19: 72–75. Article Google Scholar * Gao S, Becker B, Luo L,
Geng Y, Zhao W, Yin Y _et al_ (2016). Oxytocin, the peptide that bonds the sexes also divides them. _Proc Natl Acad Sci USA_ 113: 7650–7654. Article CAS Google Scholar * Gloy VL, Briel M,
Bhatt DL, Kashyap SR, Schauer PR, Mingrone G _et al_ (2013). Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled
trials. _Br Med J_ 347: f5934. Article Google Scholar * Gregory R, Cheng H, Rupp HA, Sengelaub DR, Heiman JR (2015). Oxytocin increases VTA activation to infant and sexual stimuli in
nulliparous and postpartum women. _Horm Behav_ 69: 82–88. Article CAS Google Scholar * Herisson FM, Waas JR, Fredriksson R, Schioth HB, Levine AS, Olszewski PK (2016). Oxytocin acting in
the nucleus accumbens core decreases food intake. _J Neuroendocrinol_ 28. (http://onlinelibrary.wiley.com/doi/10.1111/jne.12381/abstract). * Holsen LM, Lawson EA, Blum J, Ko E, Makris N,
Fazeli PK _et al_ (2012). Food motivation circuitry hypoactivation related to hedonic and nonhedonic aspects of hunger and satiety in women with active anorexia nervosa and weight-restored
women with anorexia nervosa. _J Psychiatry Neurosci_ 37: 322–332. Article Google Scholar * LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM (2001). Hunger selectively
modulates corticolimbic activation to food stimuli in humans. _Behav Neurosci_ 115: 493–500. Article CAS Google Scholar * Lawson EA, Marengi DA, DeSanti RL, Holmes TM, Schoenfeld DA,
Tolley CJ (2015). Oxytocin reduces caloric intake in men. _Obesity_ 23: 950–956. Article CAS Google Scholar * Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ (1991). Localization of
high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. _Brain Res_ 555: 220–232. Article CAS Google Scholar * Maldjian JA, Laurienti PJ,
Kraft RA, Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. _NeuroImage_ 19: 1233–1239. Article Google Scholar *
Mansouri FA, Tanaka K, Buckley MJ (2009). Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. _Nat Rev Neurosci_ 10: 141–152. Article CAS
Google Scholar * Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G _et al_ (2007). Oxytocin injected into the ventral tegmental area induces penile erection and increases
extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. _Eur J Neurosci_ 26: 1026–1035. Article Google Scholar * Mickey BJ, Heffernan
J, Heisel C, Pecina M, Hsu DT, Zubieta JK _et al_ (2016). Oxytocin modulates hemodynamic responses to monetary incentives in humans. _Psychopharmacology_ 233: 3905–3919. Article CAS Google
Scholar * Miller EK, Cohen JD (2001). An integrative theory of prefrontal cortex function. _Ann Rev Neurosci_ 24: 167–202. Article CAS Google Scholar * Mullis K, Kay K, Williams DL
(2013). Oxytocin action in the ventral tegmental area affects sucrose intake. _Brain Res_ 1513: 85–91. Article CAS Google Scholar * Nawijn L, van Zuiden M, Koch SB, Frijling JL, Veltman
DJ, Olff M (2016). Intranasal oxytocin enhances neural processing of monetary reward and loss in post-traumatic stress disorder and traumatized controls. _Psychoneuroendocrinology_ 66:
228–237. Article CAS Google Scholar * Nederkoorn C, Smulders FT, Havermans RC, Roefs A, Jansen A (2006). Impulsivity in obese women. _Appetite_ 47: 253–256. Article Google Scholar *
Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. _Cogn Affect
Behav Neurosci_ 12: 241–268. Article Google Scholar * Ogden CL, Carroll MD, Kit BK, Flegal KM (2014). Prevalence of childhood and adult obesity in the United States, 2011-2012. _J Am Med
Assoc_ 311: 806–814. Article CAS Google Scholar * Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG (1991). Oxytocin and an oxytocin agonist administered centrally
decrease food intake in rats. _Peptides_ 12: 113–118. Article CAS Google Scholar * Ott V, Finlayson G, Lehnert H, Heitmann B, Heinrichs M, Born J _et al_ (2013). Oxytocin reduces
reward-driven food intake in humans. _Diabetes_ 62: 3418–3425. Article CAS Google Scholar * Paffenbarger RS Jr, Blair SN, Lee IM, Hyde RT (1993). Measurement of physical activity to
assess health effects in free-living populations. _Med Sci Sports Exerc_ 25: 60–70. Article Google Scholar * Parent A (1996) _Carpenter's Human Neuroanatomy_9th edn. Williams &
Wilkins: Baltimore, MD, USA. Google Scholar * Paxinos G, Huang X-F (1995) _Atlas of the Human Brainstem_. Academic Press: San Diego, CA, USA. Google Scholar * Riem MM, van IMH, Tops M,
Boksem MA, Rombouts SA, Bakermans-Kranenburg MJ (2012). No laughing matter: intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter.
_Neuropsychopharmacology_ 37: 1257–1266. Article CAS Google Scholar * Saper CB, Chou TC, Elmquist JK (2002). The need to feed: homeostatic and hedonic control of eating. _Neuron_ 36:
199–211. Article CAS Google Scholar * Sellaro R, Colzato LS (2017). High body mass index is associated with impaired cognitive control. _Appetite_ 113: 301–309. Article Google Scholar *
Skuse DH, Gallagher L (2009). Dopaminergic-neuropeptide interactions in the social brain. _Trends Cogn Sci_ 13: 27–35. Article CAS Google Scholar * Sofroniew MV (1983). Morphology of
vasopressin and oxytocin neurones and their central and vascular projections. _Prog Brain Res_ 60: 101–114. Article CAS Google Scholar * Spielberger CD, Gorsuch RL, Lushene R, Vagg PR,
Jacobs GA (1983). _Manual for the Stait-Trait Anxiety Scale_. Consulting Psychologists: Palo Alto, CA, USA. * Stojek MMK, MacKillop J (2017). Relative reinforcing value of food and delayed
reward discounting in obesity and disordered eating: a systematic review. _Clin Psychol Rev_ 55: 1–11. Article Google Scholar * Striepens N, Schroter F, Stoffel-Wagner B, Maier W,
Hurlemann R, Scheele D (2016). Oxytocin enhances cognitive control of food craving in women. _Hum Brain Mapp_ 37: 4276–4285. Article Google Scholar * Stunkard AJ, Messick S (1985). The
three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. _J Psychosom Res_ 29: 71–83. Article CAS Google Scholar * Thienel M, Fritsche A, Heinrichs M,
Peter A, Ewers M, Lehnert H _et al_ (2016). Oxytocin's inhibitory effect on food intake is stronger in obese than normal-weight men. _Int J Obes_ 40: 1707–1714. Article CAS Google
Scholar * van der Klaauw AA, Ziauddeen H, Keogh JM, Henning E, Dachi S, Fletcher PC _et al_ (2017). Oxytocin administration suppresses hypothalamic activation in response to visual food
cues. _Sci Rep_ 7: 4266. Article Google Scholar * Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA _et al_ (1998). The emotional counting Stroop paradigm: a functional
magnetic resonance imaging probe of the anterior cingulate affective division. _Biol Psychiatry_ 44: 1219–1228. Article CAS Google Scholar * Wigton R, Radua J, Allen P, Averbeck B,
Meyer-Lindenberg A, McGuire P _et al_ (2015). Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. _J
Psychiatry Neurosci_ 40: E1–22. Article Google Scholar * Yanovski SZ, Yanovski JA (2014). Long-term drug treatment for obesity: a systematic and clinical review. _J Am Med Assoc_ 311:
74–86. Article CAS Google Scholar * Zhang H, Wu C, Chen Q, Chen X, Xu Z, Wu J _et al_ (2013). Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models.
_PLoS ONE_ 8: e61477. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank our participants as well as the staff at the Massachusetts General Hospital
Clinical Research Center and Athinoula A. Martinos Center for Biomedical Imaging. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Neuroendocrine Unit, Massachusetts General Hospital, Boston,
MA, USA Franziska Plessow, Dean A Marengi, Sylvia K Perry & Elizabeth A Lawson * Department of Medicine, Harvard Medical School, Boston, MA, USA Franziska Plessow & Elizabeth A
Lawson * Division of Neurotherapeutics, Massachusetts General Hospital, Charlestown, MA, USA Julia M Felicione, Rachel Franklin & Thilo Deckersbach * Clinical Research Center,
Massachusetts General Hospital, Boston, MA, USA Tara M Holmes * Division of Women’s Health, Department of Medicine and Department of Psychiatry, Brigham and Women’s Hospital, Boston, MA, USA
Laura M Holsen * Department of Psychiatry, Harvard Medical School, Boston, MA, USA Laura M Holsen, Nikolaos Makris & Thilo Deckersbach * Athinoula A. Martinos Center for Biomedical
Imaging, Charlestown, MA, USA Nikolaos Makris * Department of Psychiatry, Center for Morphometric Analysis, Massachusetts General Hospital, Boston, MA, USA, Nikolaos Makris Authors *
Franziska Plessow View author publications You can also search for this author inPubMed Google Scholar * Dean A Marengi View author publications You can also search for this author inPubMed
Google Scholar * Sylvia K Perry View author publications You can also search for this author inPubMed Google Scholar * Julia M Felicione View author publications You can also search for this
author inPubMed Google Scholar * Rachel Franklin View author publications You can also search for this author inPubMed Google Scholar * Tara M Holmes View author publications You can also
search for this author inPubMed Google Scholar * Laura M Holsen View author publications You can also search for this author inPubMed Google Scholar * Nikolaos Makris View author
publications You can also search for this author inPubMed Google Scholar * Thilo Deckersbach View author publications You can also search for this author inPubMed Google Scholar * Elizabeth
A Lawson View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Franziska Plessow. POWERPOINT SLIDES POWERPOINT SLIDE FOR
FIG. 1 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Plessow, F., Marengi, D., Perry, S. _et al._ Effects of Intranasal Oxytocin on the Blood
Oxygenation Level-Dependent Signal in Food Motivation and Cognitive Control Pathways in Overweight and Obese Men. _Neuropsychopharmacol._ 43, 638–645 (2018).
https://doi.org/10.1038/npp.2017.226 Download citation * Received: 07 July 2017 * Revised: 29 August 2017 * Accepted: 15 September 2017 * Published: 20 September 2017 * Issue Date: February
2018 * DOI: https://doi.org/10.1038/npp.2017.226 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
