The impact of combinations of alcohol, nicotine, and cannabis on dynamic brain connectivity
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Alcohol, nicotine, and cannabis are among the most commonly used drugs. A prolonged and combined use of these substances can alter normal brain wiring in different ways depending on
the consumed cocktail mixture. Brain connectivity alterations and their change with time can be assessed using functional magnetic resonance imaging (fMRI) because of its spatial and
temporal content. Here, we estimated dynamic functional network connectivity (dFNC) as derived from fMRI data to investigate the effects of single or combined use of alcohol, nicotine, and
cannabis. Data from 534 samples were grouped according to their substance use combination as controls (CTR), smokers (SMK), drinkers (DRN), smoking-and-drinking subjects (SAD), marijuana
users (MAR), smoking-and-marijuana users (SAM), marijuana-and-drinking users (MAD), and users of all three substances (ALL). The DRN group tends to exhibit decreased connectivity mainly in
areas of sensorial and motor control, a result supported by the dFNC outcome and the alcohol use disorder identification test. This trend dominated the SAD group and in a weaker manner MAD
and ALL. Nicotine consumers were characterized by an increment of connectivity between dorsal striatum and sensorimotor areas. Where possible, common and separate effects were identified and
characterized by the analysis of dFNC data. Results also suggest that a combination of cannabis and nicotine have more contrasting effects on the brain than a single use of any of these
substances. On the other hand, marijuana and alcohol might follow an additive effect trend. We concluded that all of the substances have an impact on brain connectivity, but the effect
differs depending on the dFNC state analyzed. SIMILAR CONTENT BEING VIEWED BY OTHERS HETEROGENEOUS NEUROIMAGING FINDINGS ACROSS SUBSTANCE USE DISORDERS LOCALIZE TO A COMMON BRAIN NETWORK
Article 25 September 2023 BRAIN NETWORK DYSFUNCTIONS IN ADDICTION: A META-ANALYSIS OF RESTING-STATE FUNCTIONAL CONNECTIVITY Article Open access 28 January 2022 DISRUPTED BRAIN STATE DYNAMICS
IN OPIOID AND ALCOHOL USE DISORDER: ATTENUATION BY NICOTINE USE Article 07 November 2023 INTRODUCTION The 2014 global drug survey indicates that alcohol, nicotine, and cannabis are the
three most used drugs (Winstock, 2014). Prolonged use of these substances may end in addiction, a disease surrounded by behavioral and social-context aspects influenced by biological changes
in the brain (Doyon et al, 2013; Leshner, 1997; Volkow et al, 2014). Several theories have emerged as an attempt to explain the underlying mechanism of addiction. One of these views is the
anhedonia hypothesis that the subjective pleasure induced by dopamine concentrations in the brain plays a critical role in reinforcement, motivation, and reward (Wise, 2008, 2010). Although
drugs of abuse alter dopamine concentrations in limbic brain regions, these dopaminergic alterations are not sufficient to explain the whole process of addiction (Goldstein and Volkow,
2002). For example, transitioning from voluntary to habitual drug use may be a consequence of impaired brain areas involved in executive control over behavioral inhibition processes (Everitt
and Robbins, 2005; Goldstein and Volkow, 2002). Furthermore, alterations in the mesolimbic dopamine system caused by addictive substances may be the starting point of a series of
neuroadaptations that produces changes in a neurocircuitry composed of the prefrontal cortex, cingulate, amygdala, insula, and the striatum (Koob and Volkow, 2010). These observations
promote an increasing interest in characterizing interactions among components of the proposed neurocircuitry along with the impact it might have in other brain areas. The emergence of
neuroimaging technologies facilitates the study of these interactions by providing information regarding connections among brain areas. Brain connectivity allows for the study of an
important aspect based on brain circuitry and provides additional ways to look at the complex mechanism of addiction (Pariyadath et al, 2016; Sutherland et al, 2012). Substances of abuse act
as modifiers of the dopamine-reward systems that affect the nucleus accumbens (NAc) and ventral tegmental area (VTA), but through different molecular mechanisms (Nestler, 2005). Pertinent
literature suggests that these differences translate into diverse effects in functional connectivity. Alcohol produces dysfunctions in connectivity between the NAc and other cortical areas,
including those with increased activation during stimuli and response demanding tasks (Camchong et al, 2013c). Changes in the functional connectivity of cognitive, motor, and coordination
brain areas have also been linked to alcohol use (Camchong et al, 2013b; Chanraud et al, 2011). Nicotine binding to nicotinic acetylcholine receptors (nAChR) changes dopaminergic signaling
in the areas of the reward pathway such as VTA and NAc (Subramaniyan and Dani, 2015). However, the effect of nicotine in functional connectivity is more prominent in areas like the insula
(Sutherland et al, 2012). Furthermore, nicotine administration might increase functional connectivity in motor, attention, and memory brain areas (Jasinska et al, 2014). In addition to the
reward system, cannabis also produces alterations of functional connectivity that include the orbitofrontal cortex (Filbey et al, 2014), default mode network, and insula (Pujol et al, 2014).
Evidence supports the idea that combined substance use has different effects from that of single substance use. The use of both nicotine and marijuana enhances nAChR availability in the
prefrontal cortex and the thalamus to a higher degree than single substance use (Brody et al, 2016). Reports indicate that combined use can also increase connectivity disruptions in
posterior cortical and frontoparietal regions (Jacobsen et al, 2007). Combined alcohol and nicotine consumption also produces neurologic consequences different from single substance use
(Meyerhoff et al, 2006). One important effect is that alcohol consumers are sensitive to the cognitive enhancement effects of nicotine (Ceballos et al, 2006). This work utilizes dynamic
functional network connectivity (dFNC) and multivariate approaches to investigate the different effects of alcohol, nicotine, and/or cannabis. In dFNC, connectivity is estimated at
relatively short time lengths. A small set of connectivity patterns, termed dFNC states, appear successively through time (Allen et al, 2014). Because of its fine time resolution, a dFNC
analysis results in a high dimensional data set. Given the large amount of dFNC variables influenced by substance comorbidity and important confounding problems (Meyerhoff et al, 2006), we
selected a multivariate approach as appropriate for dFNC data and poly-substance studies (Richmond-Rakerd et al, 2016). Although the nature of this dFNC study is mainly exploratory, we
expect to find previously unobserved effects or confirmatory evidence for outcomes formerly observed in functional connectivity. We also expect a disentanglement of comorbid effects as aided
by the fine timescale in dFNC analysis. MATERIALS AND METHODS SUBJECTS The sample cohort included 534 subjects (195 females) between the ages of 18 and 55 (33.2±9.7) years. Subject
exclusion criteria included injury to the brain, brain-related medical problems, and bipolar or psychotic disorders. A urinalysis test confirmed or rejected the use of drugs including
marijuana. The severity of alcohol use was assessed by applying the Alcohol Use Disorder Identification Test (AUDIT) (Saunders et al, 1993). AUDIT scores up to seven suggested abstinence and
AUDIT scores eight or larger characterized drinking status (Vergara et al, 2017b; Weiland et al, 2014). Nicotine dependence levels were assessed using the Fagerstrom Tolerance Questionnaire
(FTQ) (Fagerström, 1978). Smoking was avoided 3 h before scanning. Subjects with FTQ scores of ⩾7 were cataloged as smokers (Moore et al, 1987). Measurements of timeline follow-back (TLFB)
approach determined the level of marijuana use (60TLFB Marijuana Days). A 60TLFB of ⩾15 days defined marijuana use status. The combinations of thresholded substance use and dependence scores
were used to define eight subject groups: controls (CTR), smoking (SMK), drinking (DRN), smoking-and-drinking (SAD), marijuana (MAR), marijuana-and-drinking (MAD), smoking-and-marijuana
(SAM), and users of all three substances (ALL). The CTR group did not include AUDIT scores, but did not show alcohol abuse/dependence as assessed using the Structured Clinical Interview for
DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) (First et al, 2002). All control subjects reported no use of nicotine or marijuana. Other relevant data collected
included the Beck Depression Inventory (BDI) (Beck et al, 1988), the Beck Anxiety Inventory (Leyfer et al, 2006), the Impulsive Sensation Seeking Scale (ImpSS) (Zuckerman, 1996), and income
was used as a socioeconomic status variable. Income was categorized in 7 levels: 1: $0–$9999/year, 2: $10 000–$19 999/year, 3: $20 000–$29 999/year, 4: $30 000–$39 999/year, 5: $40 000–$49
999/year, 6: $50 000–$59 999/year, and 7: >$60,000/year. Table 1 shows detailed information about the eight groups. MRI DATA Data were collected on a 3T Siemens TIM Trio (Erlangen,
Germany) scanner. Echo-planar EPI sequence images (TR=2000 ms, TE=29 ms, flip angle=75°) were acquired with an 8-channel head coil. Volumes consisted of 33 axial slices (64 × 64 matrix, 3.75
× 3.75 mm2, 3.5 mm thickness, 1 mm gap). Data were preprocessed using the statistical parametric mapping software (SPM; http://www.fil.ion.ucl.ac.uk/spm) (Friston, 2003), including
slice-timing correction, realignment, co-registration, spatial normalization, and transformation to the Montreal Neurological Institute (MNI) standard space. Realignment parameters were
regressed out of the functional magnetic resonance imaging (fMRI) data and then smoothed using a FWHM Gaussian kernel of size 6 mm. Group ICA (Calhoun et al, 2001; Calhoun and Adali, 2012),
available through the Group ICA fMRI Toolbox (GIFT; http://mialab.mrn.org/software/gift/), was then applied to obtain a set of 100 independent components. A total of 39 out of the 100
estimated components were selected based on frequency content and visual inspection (Allen et al, 2011). In this analysis, a component is also named a resting state network (RSN) as it
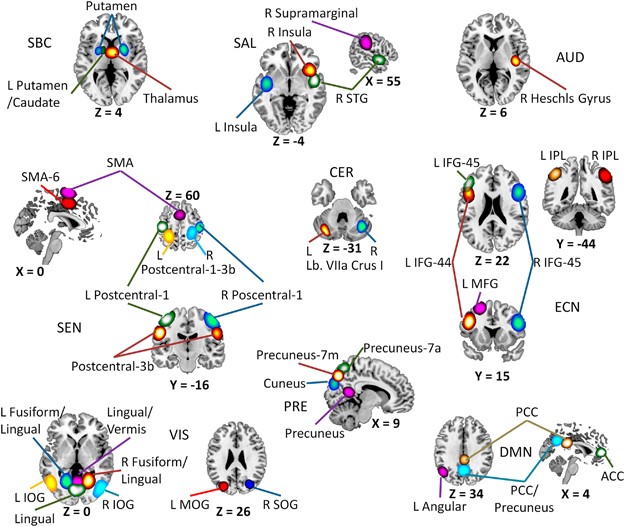
comprised a network including several areas of the brain and the fMRI scan was obtained during resting state. The 39 components were considered the RSNs of interest. Figure 1 shows the
spatial content of included RSNs. DYNAMIC FUNCTIONAL CONNECTIVITY In addition to spatial information, a set of time courses characterize the temporal evolution of each RSN. The dFNC data
were obtained using the sliding-time-window-correlation applied to RSN time courses (Louie and Wilson, 2001). Temporal window size was 40 s aiming at a fine resolution of temporal dynamics.
Windowed correlations totaled 741 for each sliding TR shift. The data were structured such that one complete set of 741 windowed correlations represent the information of a single point in
time. For simplicity, each set of 741 correlations in each TR shift will be named a window. Data from all subjects were clustered using the _k_-means clustering algorithm (Lloyd, 1982) to
estimate the set of dFNC states in the data. The number of clusters was estimated to be 6 using the GIFT software package (Rachakonda et al, 2007). Figure 2 displays centroids for all six
states where the difference in connectivity patterns is evident. RESULTS SUBSTANCES AFFECTING THE OCCUPANCY RATES OF DFNC STATES Subject-wise occupancy rates were determined by the frequency
with which each dFNC state appeared on each subject. Occupancy rate differences for each of the eight substance groups were then tested utilizing six one-way ANOVAs. The ANOVA tests for
state 2 (F=2.49, _P_=0.0161) and state 4 (F=2.47, _P_=0.0170) were significant. ANOVA tables are provided in Supplementary Material. Figure 3 displays mean occupancy rates and _post hoc_
results from Fisher’s least significant difference (LSD) (Fisher, 1937). After detecting a significant ANOVA, LSD finds the smallest significant difference and considers larger differences
as significant (Williams and Abdi, 2010). The DRN group showed larger occupancy than CTR and ALL groups in state 4. The MAR group also showed larger occupancy than the ALL group. In
contrast, DRN and MAD groups had lower occupancy than the CTR group in state 2. The SAM group had larger occupancy rate than DRN. In both states, MAR and DRN groups follow the similar
tendency with high occupancy in state 2 and low occupancy in state 3, but because of its large variance the MAR group had no significant differences with other groups in state 2. A linear
regression model was applied with the occupancy rate as dependent variable (DV). Results from linear regression models displayed in Table 2 show a significant and negative slope between
occupancy rates and AUDIT in state 2, agreeing with the ANOVA _post hoc_ analysis in Figure 3. However, state 4 had no significant regression coefficients of substance-related scores. There
were no significant coefficients for depression (BDI), anxiety (BAI), and sensation seeking (ImpSS) on any of the six states. State 2 had significant relationships with sex and income, but
AUDIT had the larger effect size. The largest covariate in state 4 was age with a very strong effect size, followed by income as the second strongest covariate. Movement noise on the
_z_-axis was significant in state 4 and might have contributed to the lack of effects related to substances. The strongest result indicates that drinkers and comorbid alcohol–cannabis users
exhibit a reduced influence in state 2, where connectivity among saliency, motor, and sensorial processing is strong. Connectivity for the cannabis group follows a similar trend, but the
lack of significance may be influenced by the differences in sample sizes. Nicotine and comorbid nicotine–cannabis did not show an effect or a trend in this occupancy rate analysis.
SUBSTANCE EFFECTS IN DFNC STRENGTH A multivariate analysis of variance (MANOVA) (Warne, 2014) was used in each state to determine group differences using correlations from all 741 (39 ×
38/2) RSN pairs as DVs. Windows belonging to the same dFNC state were averaged for each subject to represent the subject on a particular state. Within a dFNC state, the 741 average values of
each subject entered MANOVA as DVs. MANOVA results were all significant (_P_<1e–94) indicating at least one group difference. Specifics about the MANOVA results are included in the
Supplementary Material. The _post hoc_ analyses were performed using linear discriminant analysis (LDA). LDA is a multivariate method, a generalization of Fisher’s linear discriminant
(Iatan, 2010), used to find linear combinations of DVs that best separate the groups. In our context, LDA delivers a finite set of vectors (termed canonical vectors) with subject
corresponding values. For ease of illustration, we only utilized the first two canonical vectors for the scatter plots displayed in Figure 4 to show group separation in a two-dimensional
space. The scatter plot also includes a vector indicating the direction of increasing connectivity strength. Connectivity strength is a global measure of connectivity obtained by averaging
all available correlations (Lynall et al, 2010). The global characteristic of connectivity strength is appropriate for this analysis given that LDA is a linear combination of connectivity
values spanning the whole brain. The connectivity strength vector was obtained by averaging the 741 dFNCs on each state and calculating the projection to the corresponding canonical vectors.
In this analysis, only the vector direction is important and points to increasing dFNC connectivity strength. Two dimension scatter plots illustrate subject group segregation where all
eight groups are almost perfectly separated. However, this visualization does not show dFNC information of all seven dimensions. Figure 4h exhibits the distribution of subject groups along
the projection of the connectivity strength vector in seven dimensions. The way this projection was calculated is exemplified in Figure 4g. Figure 4h allows for the visualization of the
relationship between subject group and connectivity strength. The results illustrate the possibility of discriminating between the eight groups by means of a multivariate analysis and
performing specific linear combinations of dFNC values. In state 1, the MAR and ALL groups show larger connectivity strength than the rest, but the SAM group exhibits a lower value. In
states 2, 3, and 5, the MAR group has noticeably larger connectivity strength than the others. In state 2, CTR and SAM groups have the second strongest connectivity strength. Besides the MAR
group, the other subject groups do not differentiate along the connectivity strength axis. State 4 exhibits a SAM group with larger connectivity strength than the other groups. State 5 has
the most confounded subject groups of all states. State 6 shows low connectivity strength in the SAM and DRN groups; the CTR group has higher connectivity strength than the other groups.
Because canonical vectors are linear combinations of the dFNCs, it is difficult to see changes in connectivity for single RSN pairs. Specific connectivity effects were analyzed using ANOVAs
on dFNC values (one ANOVA for each one of the 741 dFNCs) seeking significant group differences (_P_<0.05) and correcting using false discovery rate (FDR). Finally, a linear regression
model was applied to the subset of 741 connectivity values with significant ANOVA. The linear model assumed the connectivity between a particular RSN pair as DV. The significant ANOVA
results are displayed in Figure 5 and regression model results are presented in Table 3. Movement covariates based on the mean frame-wise displacement of each coordinate were included to
correct and diminish possible movement variance in the analysis. Only the result from state 1 had a significant noise related to the _y_-axis movement, but the inclusion of this covariate
reduced the influence of movement. The other results in Table 3 did not exhibit a significant movement variance. Group results for states 1 and 2 were consistent with a reduction of dFNC
because of alcohol. In both states, the DRN group presented a lower dFNC mean with respect to CTR and SMK groups. At the same time, both states present a significant negative slope with
AUDIT increasing the evidence that alcohol use is related to this diminished connectivity. The result from state 1 feature connectivity between the left inferior frontal gyrus (L. IFG) and
the right postcentral gyrus. The particularity of this result is a significant relationship with cannabis consumption. It might explain in part the increased mean dFNC in the ALL group and
the difference between DRN and MAD groups. The result from state 2 include the connectivity between a RSN comprising right fusiform and lingual giri, and a RSN with supplementary motor area
(SMA) content. The relationship between CTR, DRN, and SMK groups is the same as in state 1. The difference can be seen in a lack of a significant marijuana link and the ALL group exhibits a
decreased dFNC. For the ALL group, alcohol use seems to lead the direction of the effect in state 2 as opposed to the strong influence of marijuana use seen in state 1. Four ANOVAs were
significant in state 4 involving the connectivity between dorsal striatum and sensorimotor regions. Groups of nicotine use (SMK, SAD, SAM, and ALL) had no significant difference in dFNC for
the pair putamen and SMA. With some few exceptions, these four nicotinic groups exhibited a lower dFNC than the nonnicotine groups including CTR. The regression analysis reveals a strong
effect (22.9% of variance explained) of reduced connectivity with increased FTQ. These results suggest a strong connectivity reduction between putamen and SMA linked to nicotine use. An
almost identical pattern can be observed for the RSN pair L.Putamen/Caudate and L.Postcentral-1-3b where the effect size for FTQ is also significant (10% of variance explained). Although
similar in trend and anatomical regions, the other two RSN pairs show a significant link with nicotine use. However, these results exhibited a lack of group difference between CTR and SMK
groups. Except for a significant link to AUDIT in the pair L.Putamen/Caudate _vs_ L.Postcentral-1, no other covariate had a significant effect on dFNC. The dFNC in state 4 seems to be mainly
influenced by nicotine use. DISCUSSION Drawing from the poly-substance characteristics of the subjects considered in this study, one important objective of this work was to detect dFNC
differences linked to combinations of the three commonly used substances. In general, the data were rich enough to allow for a match of substance use and dFNC characteristics acquired
through appropriate linear combinations. These linear combinations do not point to a particular gray matter region, but represent the aggregated contribution of brain areas spanning the
whole brain. Figure 4a–f illustrates 2 out of the 7 available data dimensions with significant information about differences in the 741 dFNC measures. These scatter plots show that each
substance or their combined use has separate and identifiable effects in the brain. The difficulty in interpreting MANOVA results comes from its multivariate and higher dimensional features.
As an outcome of the aggregation of connectivity through the whole brain, it is reasonable to compare the results with a global measure of connectivity. Figure 4h presents an interpretable
visualization of the results based on global connectivity strength measure. Connectivity differences could be seen at the global level that we discuss in the following paragraphs along with
more localized effects. The overall effect of alcohol is a reduction of dFNC, especially among motor and sensorial areas. Figure 3 shows evidence that alcohol drinkers avert state 2, where
there is a strong connectivity among sensorimotor, salience, and precuneus brain areas. The lack of preference for state 2 is confirmed by the negative regression coefficient that was found
significant for the occupancy rate of state 2 in Table 2. The connectivity pattern in state 2 is in line with the hypothesis of an extrospective mind state within the resting state,
providing readiness in case attention to outside stimuli is necessary (Fransson, 2005). In contrast, there is an inflated preference for state 4, where the mentioned brain areas are weakly
connected. Not only the dFNC state preference is different for drinkers, but also connectivity strength. Figure 4h presents additional connectivity reduction in most states including states
2 and 4. In addition to whole brain connectivity data, univariate analyses identified reduced dFNC in drinkers between visual and motor areas, agreeing with the multivariate outcomes. Linear
model results in this study provide previously missing evidence (Vergara et al, 2017a) of a significant link between alcohol effects in functional connectivity with alcohol use measures;
specifically, the AUDIT score. This set of consistent results observed in dFNC also agrees with former studies of static functional connectivity. A previous study found a reduction of static
functional connectivity primarily among the insula, precuneus, sensorimotor, and visual areas, but an increase on the putamen after testing for group differences against nondrinkers
(Vergara et al, 2017a). These outcomes support both the ‘disconnection syndrome’ (Dupuy and Chanraud, 2016) and the reduced interoception effect (Çöl et al, 2016) related to alcohol use.
Alcohol-related functional disconnection has been reported by several studies (Weiland et al, 2014) as associated with drinking, abstinence, and relapse (Camchong et al, 2013a, b) and loss
of network efficiency in the brain (Sjoerds et al, 2017). This and the previously mentioned studies only presented evidence of functional disconnection, but structural studies have also
indicated decreased white matter integrity (Jansen et al, 2015; Kril et al, 1997; Yeh et al, 2009); this helps explain the overall extent of the disconnection syndrome. One important point
is that observed functional disconnection is more prominent in state 2, where salient brain areas in the insula (see the insula in Figure 1 and state 2 in Figure 2) are strongly connected
with other brain regions. The insula is actively involved in interoception because it is a structure that processes the physiological condition of the rest of the body (Craig, 2003). The
results are in accordance with the idea of a reduced interoception because of the disconnection produced by alcohol use. Evidence of reduced iteroception awareness related to alcohol use has
been previously presented (Çöl et al, 2016) by means of a heartbeat perception performance method. In addition to alcohol, our data suggest that marijuana consumption may produce aberrant
interoception patterns because of an increased occupancy rate in state 4 (see Figure 3) where the insula is weakly connected to the rest of the brain. Connectivity strength was also reduced
in marijuana subjects within state 4 (see Figure 4); however, state 2 presents the opposite effect with different outcome than alcohol drinkers. It has been theorized that aberrant
interoception is an effect that belongs to all addiction in general (Verdejo-Garcia et al, 2012). In this respect, our data suggest that alcohol may produce one of the largest aberrant
interoception among the substances of abuse. We can turn our attention to state 1, where univariate analysis found a decrease in dFNC between a frontal area of the ECN and postcentral gyrus;
both task-positive networks (TPNs). TPNs are brain networks elicited to perform demanding tasks (Fox et al, 2005). Aberrant connectivity in the ECN has been suggested as a contributing
factor in sustaining alcohol addiction (Weiland et al, 2014). Group differences found in our analyses agree in part with predictions of the network model of addiction where resting state
connectivity among TPNs and between salience and TPNs are reduced after substance use (Sutherland et al, 2012). In summary, alcohol use produces a general resting state functional
disconnection that is harsh on TPNs (including sensorimotor and executive control areas) and the insula, a region important for interoceptive functions. Concurrent nicotine and alcohol
consumption did not have an effect in occupancy rates. The SAD group exhibited similar connectivity strength as the DRN group in states 2 and 4. There was no similar trend between SMK and
SAD groups. In states 1 and 3, the two groups had connectivity strength similar to controls indicating the absence of an important effect. The similarity of effect between populations that
drink and those that concurrently smoke and drink has been observed before in studying static FNC (Vergara et al, 2017a). In that static FNC study, there was a sparing of high visual areas
in SAD subjects. A similar outcome was observed in Figure 5, where the connectivity between motor and a high visual processing area was reduced in drinkers, but was unaffected in SAD
subjects. This outcome could be related to the activation enhancement of high visual areas produced by nicotine (Ghatan et al, 1998; Lawrence et al, 2002). The effect of nicotine on alcohol
drinkers might have diminished dysfunctional connectivity because of alcohol, but future research is needed to verify the existence of this effect. Nicotine outcomes were fewer overall, but
with some relatively large effect sizes. Although no group differences in occupancy rate were found in relation to the SMK group, significant associations with FTQ were observed in the
linear model. Higher occupancy rates in state 5 were linked to larger FTQ values. Lower occupancy rates in state 6 were associated with larger FTQ. The main difference between these two
states is the higher connectivity of the ECN and the DMN in state 6 as compared with state 5, suggesting that nicotine reduces connectivity of the ECN and the DMN. Connectivity strength
results in Figure 4 show a tendency for connectivity reduction in states 2, 5, and 6 when comparing SMK and CTR groups, favoring a reduced connectivity because of nicotine use. However,
connectivity strength has a contrasting picture of increased connectivity in state 3 and no difference with controls in states 1 and 4. These contrasting results are not completely
unexpected as nicotine has been found to produce both increased and decreased connectivity in some brain areas, including the frontoparietal network and the DMN (Pariyadath et al, 2014).
Univariate analysis produced a more conclusive set of outcomes. ANOVA results in Figure 5 show a significant dFNC decrease in smokers compared with controls between sensorimotor and dorsal
striatum areas in state 4. Reduced dFNC in nicotine users is further verified by significant links between FTQ and striatal-sensorimotor connectivity displayed in Table 3 with large effect
sizes characterized by percentages of variance explained between 13% and 22%. To the best knowledge of the authors, this is one of the few times a strong effect of resting state functional
connectivity has been observed in the dorsal striatum linked to nicotine. Static connectivity analysis using seed-based methods found that smokers during an abstinent period of 24 h exhibit
decreased connectivity between dorsal striatum and cortical regions that include the supplementary motor area (Sweitzer et al, 2016). Although the ventral striatum is more frequently
associated with nicotine addiction because of its role in the dopamine pathway (Brody et al, 2004; Okita et al, 2016), the dorsal striatum is thought to become a more important player as
drug seeking transitions from voluntary to habitual behavior (Everitt and Robbins, 2005). This transition has been suggested to be present in abstinent nicotine smokers as an underlying
mechanism that suppresses some automated habitual conduct in favor of diverting resources to craving and nicotine seeking behavior (Sweitzer et al, 2016; Tiffany and Conklin, 2000). There is
also evidence that the dorsal, and not the ventral, striatum suffer morphological changes (volume and surface area) associated with nicotine craving (Janes et al, 2015). Our data and the
previously mentioned studies support the existence of structural and functional connectivity changes in the dorsal striatum (putamen and caudate) linked to nicotine use and dependence. The
connectivity strength of marijuana subjects was larger than controls in states 1, 2, 3, and 5, but lower with a smaller magnitude in the other two states as displayed in Figure 4h. This
result indicates that marijuana induces a stronger increment of connectivity through the brain, in selected dFNC states, than decrements. The effects were observed in a whole brain
connectivity summary, but were not observable when selecting specific brain areas. Figure 5 illustrates this observation in the boxes comparing the CTR group with the rest. It can be argued
that two factors contributed to the small number of marijuana results: (1) the large number of comparisons that were corrected and (2) the small number of marijuana subjects. Nevertheless,
multivariate group results were observed as they are based on the linear combination of contributions from many group differences that were excluded if applying statistical multicomparison
correction. Increased functional connectivity in cannabis users compared with controls has been previously reported in areas including the orbitofrontal cortex (Filbey et al, 2014);
precentral, middle frontal, superior frontal, cingulate, inferior frontal, and fusiform giri (Cheng et al, 2014); and posterior cingulate and insula (Pujol et al, 2014). These observations
of functional connectivity increments, including that in our data, are not expressions of beneficial effects. Structural studies found a series of axonal impairment in the hippocampus
(fornix), splenium, commissural fibers (Zalesky et al, 2012), and morphological changes in the amygdala (Cousijn et al, 2012), cerebellum (Cousijn et al, 2012; Medina et al, 2010), and
prefrontal cortex (Medina et al, 2009). One hypothesis that can explain why increased functional connectivity might point to an actual dysfunction suggests interference of some brain network
in the normal function of others (Sutherland et al, 2012). This idea has been used to explain increased connectivity in key areas of the default mode network as a detrimental effect on the
brain (Pujol et al, 2014). As previously explained, both marijuana and alcohol consumptions are linked to changes of structural connectivity (Jansen et al, 2015; Zalesky et al, 2012), but
affect functional connectivity in opposite directions as alcohol decreases (Camchong et al, 2013b; Vergara et al, 2017a; Weiland et al, 2014) and marijuana increases (Cheng et al, 2014;
Filbey et al, 2014; Pujol et al, 2014) overall connectivity. Thinking in an additive way, the effect of concurrent use of these substances may subtract each other and this trend should be
observable in the MAD sample group. Connectivity strength measures in Figure 4h support this view, giving the trend of the MAD group to be closer to controls (CTR group) in states 2 and 5,
where the MAR group has increased but the DRN group decreased connectivity. Univariate ANOVA results in Figure 5 show a MAD group with higher connectivity than the DRN group in state 1,
suggesting that alcohol reduced connectivity between postcentral and inferior frontal gyrus, but mixed alcohol and marijuana diminished the effect of alcohol. A similar trend in higher
connectivity in MAD _vs_ DRN groups is observed in state 4. Increased connectivity in MAD compared with DRN groups could in part be explained by reported increments of structural
connectivity in the prefrontal cortex linked to marijuana use (Filbey et al, 2014). More evidence can be found comparing the brain of adolescents, where subjects that binge drink and consume
marijuana exhibited less white matter alterations than those who only consumed alcohol (Jacobus et al, 2009). Even though a subtractive effect is plausible, the consequences do not
translate into beneficial outcomes. Comorbid alcohol and marijuana consumption does have a toll in neurocognitive abilities including verbal learning, memory, attention, processing speed,
visuospatial functioning, and cognitive control (Squeglia and Gray, 2016). The opposite trend between alcohol and cannabis is not an indication that detrimental neurocognitive effects will
diminish because of concurrent use. The results obtained for combined nicotine and marijuana show connectivity effects that contrast with single substance use. Connectivity strength in state
4 is dramatically increased compared with all of the other samples groups. In states 1 and 6, the connectivity strength of the SAM group is lower than all other groups. These connectivity
differences do not follow an obvious trend, nor an additive effect, when compared with the MAR and SMK groups. The chemistry of combined marijuana nicotine use is characterized by an
increase of nicotinic acetylcholine receptor (nAChR) availability in the prefrontal cortex and the thalamus as compared with single nicotine use (Brody et al, 2016). In the same work, this
interaction thought to occur at the cell molecular level was also found in mixed nicotine caffeine consumption. Availability of nAChRs modulate whole brain connectivity measures such as
global network efficiency that measures the efficiency of information transfer through the brain (Wylie et al, 2012). In similar fashion, chemical interactions of nicotine and marijuana may
have potentiated the variety of global connectivity strength effects that are seen in Figure 4h. These outcomes must be interpreted in the context of whole brain analysis and cannot be used
to describe more specific effects of each substance. Univariate ANOVA outcomes of state 4 (see Figure 5) are compatible with a difference; specifically, an increment of connectivity in
comorbid marijuana and nicotine use as compared with single substance use. However, the SAM group did not show differences with controls, indicating that observed combined _vs_ single use
effects are not simple to explain. Up to this point, we can observe that some states are more affected by certain substances. For example, alcohol has a consistently strong influence in
state 2. Marijuana produced a large increase of connectivity strength in states 1, 2, and 3. Nicotine produced a large effect size between dorsal striatum and sensorimotor areas in state 4.
We can observe that the ALL group influenced by all three substances might follow the trend of one of the three substances on different states. In state 1, ALL and MAR groups had a similar
connectivity strength. This increment in the ALL could also be seen in the univariate results for state 1 (shown in Figure 5) and is an opposite effect to the decrease connectivity in the
DRN group in that state. If the interaction of marijuana and alcohol can be thought of as additive, then marijuana could have a stronger influence than alcohol in that state. Both
multivariate and univariate results agree that alcohol is the stronger influence in state 2. In Figure 4h, the ALL group may not have achieved the same decrement of connectivity strength as
that seen with the SAD and DRN groups because of the influence of marijuana. It is noteworthy that the MAR group had a very strong increment of connectivity strength. Note that ALL and MAD
groups showed similar connectivity strength. The univariate results for state 2 in Figure 5 are consistent with a decrement of connectivity in the DRN and ALL groups, suggesting that alcohol
was the most influencing substance. With respect to nicotine, only the univariate results show a consistent similarity between ALL and SMK groups in state 4, suggesting that nicotine was
the most influential substance. The effect was strong in specific areas of the brain, dorsal striatum, and sensorimotor areas, but was not observed when analyzing the connectivity strength.
We observed effects that were more focused than global effects related to nicotine. An important limitation of this study was the disparity on the number of samples, where there is a
relatively large number of alcohol users, but a low number of marijuana users. Although the low number of marijuana users allowed the observation of effects using MANOVA, the low statistical
power was more evident when analyzing single dFNCs. Many univariate results with marijuana effects were excluded after multicomparison correction, but the _P_-values were close to being
significant after FDR. Unfortunately, subjects suitable for single marijuana consumption are difficult to find and, along with the need for correcting over a large amount of comparisons,
causes a considerable limitation of statistical power. Nevertheless, multivariate analysis picked up strong signals from whole brain marijuana effects because it combined the available
information. The MAR group shows similar reduction of occupancy rate with the DRN and MAD subjects in state 2, but this reduction did not achieve significance mainly because of the small
number of subjects in MAR. However, the observed trend is compatible among these three sample groups, indicating that alcohol and cannabis might have the same effects on the occupancy rate
of state 2. In state 4, the MAR group exhibits an even higher mean occupancy than DRN (group that differ from CTR), but high variability of dFNC from MAR samples decremented detection power.
The second important limitation was the lack of covariate measures for the CTR group. Although AUDIT was missing from this group, several studies indicate that AUDIT and DSM-IV have similar
specificity (Dawson et al, 2012; Foxcroft et al, 2015), providing evidence that subjects in the CTR group were correctly classified. However, the same cannot be inferred for the other
important measures of BID, BAI, ImpSS, and Income. For this reason, linear correlation analysis was limited to the substance user. Third, the small number of single dFNC findings likely
reflects only the strongest effects. Other existing effects, such as those observed by hypotheses-driven techniques (Chanraud et al, 2011; Janes et al, 2012), may be missed. The small number
within the MAR group plays a role in this limitation. FUNDING AND DISCLOSURE The authors declare no conflict of interest. REFERENCES * Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T,
Calhoun VD (2014). Tracking whole-brain connectivity dynamics in the resting state. _Cereb Cortex_ 24: 663–676. Article PubMed Google Scholar * Allen EA, Erhardt EB, Damaraju E, Gruner W,
Segall JM, Silva RF _et al_ (2011). A baseline for the multivariate comparison of resting-state networks. _Front Syst Neurosci_ 5: 2. PubMed PubMed Central Google Scholar * Beck AT,
Steer RA, Carbin MG (1988). Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. _Clin Psychol Rev_ 8: 77–100. Article Google Scholar * Brody AL,
Hubert R, Mamoun MS, Enoki R, Garcia LY, Abraham P _et al_ (2016). Nicotinic acetylcholine receptor availability in cigarette smokers: effect of heavy caffeine or marijuana use.
_Psychopharmacology_ 233: 3249–3257. Article CAS PubMed PubMed Central Google Scholar * Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P _et al_ (2004). Smoking-induced
ventral striatum dopamine release. _Am J Psychiatry_ 161: 1211–1218. Article PubMed Google Scholar * Calhoun V, Adali T, Pearlson G, Pekar J (2001). A method for making group inferences
from functional MRI data using independent component analysis. _Hum Brain Mapp_ 14: 140–151. Article CAS PubMed PubMed Central Google Scholar * Calhoun VD, Adali T (2012). Multisubject
independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. _IEEE Rev Biomed Eng_ 5: 60–73. Article PubMed PubMed Central Google
Scholar * Camchong J, Stenger A, Fein G (2013a). Resting-state synchrony during early alcohol abstinence can predict subsequent relapse. _Cereb Cortex_ 23: 2086–2099. Article PubMed
Google Scholar * Camchong J, Stenger A, Fein G (2013b). Resting‐state synchrony in long‐term abstinent alcoholics. _Alcohol Clin Exp Res_ 37: 75–85. Article PubMed Google Scholar *
Camchong J, Stenger VA, Fein G (2013c). Resting‐state synchrony in short‐term versus long‐term abstinent alcoholics. _Alcohol Clin Exp Res_ 37: 794–803. Article CAS PubMed PubMed Central
Google Scholar * Ceballos NA, Tivis R, Lawton-Craddock A, Nixond SJ (2006). Nicotine and cognitive efficiency in alcoholics and illicit stimulant abusers: implications of smoking
cessation for substance users in treatment. _Subst Use Misuse_ 41: 265–281. Article PubMed Google Scholar * Chanraud S, Pitel A-L, Pfefferbaum A, Sullivan EV (2011). Disruption of
functional connectivity of the default-mode network in alcoholism. _Cereb Cortex_ 21: 2272–2281. Article PubMed PubMed Central Google Scholar * Cheng H, Skosnik PD, Pruce BJ, Brumbaugh
MS, Vollmer JM, Fridberg DJ _et al_ (2014). Resting state functional magnetic resonance imaging reveals distinct brain activity in heavy cannabis users - a multi-voxel pattern analysis. _J
Psychopharmacol_ 28: 1030–1040. Article CAS PubMed PubMed Central Google Scholar * Çöl IA, Sönmez MB, Vardar ME, Köşesi H (2016). Evaluation of interoceptive awareness in
alcohol-addicted patients. _Evaluation_ 53: 17–22. Google Scholar * Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE (2012). Grey matter alterations
associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. _Neuroimage_ 59: 3845–3851. Article PubMed Google Scholar * Craig A (2003).
Interoception: the sense of the physiological condition of the body. _Curr Opin Neurobiol_ 13: 500–505. Article CAS PubMed Google Scholar * Dawson DA, Smith SM, Saha TD, Rubinsky AD,
Grant BF (2012). Comparative performance of the AUDIT-C in screening for DSM-IV and DSM-5 alcohol use disorders. _Drug Alcohol Depend_ 126: 384–388. Article PubMed PubMed Central Google
Scholar * Doyon WM, Thomas AM, Ostroumov A, Dong Y, Dani JA (2013). Potential substrates for nicotine and alcohol interactions: a focus on the mesocorticolimbic dopamine system. _Biochem
Pharmacol_ 86: 1181–1193. Article CAS PubMed PubMed Central Google Scholar * Dupuy M, Chanraud S (2016). Imaging the addicted brain: alcohol. _Int Rev Neurobiol_ 129: 1–31. Article CAS
PubMed Google Scholar * Everitt BJ, Robbins TW (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. _Nat Neurosci_ 8: 1481–1489. Article CAS
PubMed Google Scholar * Fagerström K-O (1978). Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. _Addict Behav_ 3: 235–241.
Article PubMed Google Scholar * Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A _et al_ (2014). Long-term effects of marijuana use on the brain. _Proc Natl Acad Sci USA_
111: 16913–16918. Article CAS PubMed PubMed Central Google Scholar * First MB, Spitzer RL, Gibbon M, Williams JBW (2002) _Structured Clinical Interview for DSM-IV-TR Axis I Disorders,
Research Version, Patient Edition_. State Psychiatric Institute: New York. Google Scholar * Fisher RA (1937) _The Design of Experiments_. Oliver and Boyd: Edinburgh; London. Google Scholar
* Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. _Proce Natl Acad
Sci USA_ 102: 9673–9678. Article CAS Google Scholar * Foxcroft DR, Smith L, Thomas H, Howcutt S (2015). Accuracy of Alcohol Use Disorders Identification Test (AUDIT) for detecting problem
drinking in 18–35 year-olds in England. _Viitattu_ 21: 2015. Google Scholar * Fransson P (2005). Spontaneous low‐frequency BOLD signal fluctuations: an fMRI investigation of the
resting‐state default mode of brain function hypothesis. _Hum Brain Mapp_ 26: 15–29. Article PubMed PubMed Central Google Scholar * Friston KJ (2003) Statistical Parametric Mapping. In:
Kötter R (eds). _Neuroscience Databases_. Springer, Boston, MA. Google Scholar * Ghatan P, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K _et al_ (1998). Cerebral effects of
nicotine during cognition in smokers and non-smokers. _Psychopharmacology_ 136: 179–189. Article CAS PubMed Google Scholar * Goldstein RZ, Volkow ND (2002). Drug addiction and its
underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. _Am J Psychiatry_ 159: 1642–1652. Article PubMed PubMed Central Google Scholar * Iatan
IF (2010) The Fisher's linear discriminant _Advances in Intelligent and Soft Computing Combining Soft Computing and Statistical Methods in Data Analysis Berlin_. Springer-Verlag Berlin:
Berlin, Germany, pp 345–352. Book Google Scholar * Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE (2007). Functional correlates of verbal memory deficits emerging during
nicotine withdrawal in abstinent adolescent cannabis users. _Biol Psychiatry_ 61: 31–40. Article CAS PubMed Google Scholar * Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR,
Yang TT _et al_ (2009). White matter integrity in adolescents with histories of marijuana use and binge drinking. _Neurotoxicol Teratol_ 31: 349–355. Article CAS PubMed PubMed Central
Google Scholar * Janes AC, Nickerson LD, Kaufman MJ (2012). Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and
non-smoking controls. _Drug Alcohol Depend_ 125: 252–259. Article CAS PubMed PubMed Central Google Scholar * Janes AC, Park MT, Farmer S, Chakravarty MM (2015). Striatal morphology is
associated with tobacco cigarette craving. _Neuropsychopharmacology_ 40: 406–411. Article PubMed Google Scholar * Jansen JM, van Holst RJ, van den Brink W, Veltman DJ, Caan MW, Goudriaan
AE (2015). Brain function during cognitive flexibility and white matter integrity in alcohol-dependent patients, problematic drinkers and healthy controls. _Addict Biol_ 20: 979–989. Article
PubMed Google Scholar * Jasinska AJ, Zorick T, Brody AL, Stein EA (2014). Dual role of nicotine in addiction and cognition: a review of neuroimaging studies in humans.
_Neuropharmacology_ 84: 111–122. Article CAS PubMed Google Scholar * Koob GF, Volkow ND (2010). Neurocircuitry of addiction. _Neuropsychopharmacology_ 35: 217–238. Article PubMed
Google Scholar * Kril JJ, Halliday GM, Svoboda MD, Cartwright H (1997). The cerebral cortex is damaged in chronic alcoholics. _Neuroscience_ 79: 983–998. Article CAS PubMed Google
Scholar * Lawrence NS, Ross TJ, Stein EA (2002). Cognitive mechanisms of nicotine on visual attention. _Neuron_ 36: 539–548. Article CAS PubMed Google Scholar * Leshner AI (1997).
Addiction is a brain disease, and it matters. _Science_ 278: 45–47. Article CAS PubMed Google Scholar * Leyfer OT, Ruberg JL, Woodruff-Borden J (2006). Examination of the utility of the
Beck Anxiety Inventory and its factors as a screener for anxiety disorders. _J Anxiety Disord_ 20: 444–458. Article PubMed Google Scholar * Lloyd S (1982). Least squares quantization in
PCM. _IEEE Trans Inform Theory_ 28: 129–137. Article Google Scholar * Louie K, Wilson MA (2001). Temporally structured replay of awake hippocampal ensemble activity during rapid eye
movement sleep. _Neuron_ 29: 145–156. Article CAS PubMed Google Scholar * Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U _et al_ (2010). Functional connectivity and
brain networks in schizophrenia. _J Neurosci_ 30: 9477–9487. Article CAS PubMed PubMed Central Google Scholar * Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF (2009).
Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. _Addict Biol_ 14: 457–468. Article PubMed PubMed Central Google Scholar * Medina KL, Nagel
BJ, Tapert SF (2010). Abnormal cerebellar morphometry in abstinent adolescent marijuana users. _Psychiatry Res_ 182: 152–159. Article PubMed PubMed Central Google Scholar * Meyerhoff DJ,
Tizabi Y, Staley JK, Durazzo TC, Glass JM, Nixon SJ (2006). Smoking comorbidity in alcoholism: neurobiological and neurocognitive consequences. _Alcohol Clin Exp Res_ 30: 253–264. Article
CAS PubMed Google Scholar * Moore BL, Schneider JA, Ryan JJ (1987). Fagerstrom's tolerance questionnaire: clarification of item and scoring ambiguities. _Addict Behav_ 12: 67–68.
Article CAS PubMed Google Scholar * Nestler EJ (2005). Is there a common molecular pathway for addiction? _Nat Neurosci_ 8: 1445–1449. Article CAS PubMed Google Scholar * Okita K,
Mandelkern MA, London ED (2016). Cigarette use and striatal dopamine D2/3 receptors: possible role in the link between smoking and nicotine dependence. _Int J Neuropsychopharmacol_ 19: 1–5.
Google Scholar * Pariyadath V, Gowin JL, Stein EA (2016). Resting state functional connectivity analysis for addiction medicine: From individual loci to complex networks. _Prog Brain Res_
224: 155–173. Article PubMed Google Scholar * Pariyadath V, Stein EA, Ross TJ (2014). Machine learning classification of resting state functional connectivity predicts smoking status.
_Front Hum Neurosci_ 8: 425. Article PubMed PubMed Central Google Scholar * Pujol J, Blanco-Hinojo L, Batalla A, Lopez-Sola M, Harrison BJ, Soriano-Mas C _et al_ (2014). Functional
connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. _J Psychiatr Res_ 51: 68–78. Article PubMed Google Scholar * Rachakonda S, Egolf E, Correa
N, Calhoun V (2007). Group ICA of fMRI toolbox (GIFT) manual. https://www.nitrc.org/docman/view.php/55/295/v1_203d_GIFTManual.pdf_(cited 5 November 2011)_. * Richmond-Rakerd LS, Slutske WS,
Lynskey MT, Agrawal A, Madden PA, Bucholz KK _et al_ (2016). Age at first use and later substance use disorder: shared genetic and environmental pathways for nicotine, alcohol, and
cannabis. _J Abnorm Psychol_ 125: 946. Article PubMed PubMed Central Google Scholar * Saunders JB, Aasland OG, Babor TF, Grant M (1993). Development of the alcohol use disorders
identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption‐II. _Addiction_ 88: 791–804. Article CAS PubMed Google Scholar *
Sjoerds Z, Stufflebeam SM, Veltman DJ, Van den Brink W, Penninx BW, Douw L (2017). Loss of brain graph network efficiency in alcohol dependence. _Addict Biol_ 22: 523–534. Article PubMed
Google Scholar * Squeglia LM, Gray KM (2016). Alcohol and drug use and the developing brain. _Curr Psychiatry Rep_ 18: 46. Article PubMed PubMed Central Google Scholar * Subramaniyan M,
Dani JA (2015). Dopaminergic and cholinergic learning mechanisms in nicotine addiction. _Ann NY Acad Sci_ 1349: 46–63. Article CAS PubMed Google Scholar * Sutherland MT, McHugh MJ,
Pariyadath V, Stein EA (2012). Resting state functional connectivity in addiction: Lessons learned and a road ahead. _Neuroimage_ 62: 2281–2295. Article PubMed Google Scholar * Sweitzer
MM, Geier CF, Addicott MA, Denlinger R, Raiff BR, Dallery J _et al_ (2016). Smoking abstinence-induced changes in resting state functional connectivity with ventral striatum predict lapse
during a quit attempt. _Neuropsychopharmacology_ 41: 2521–2529. Article PubMed PubMed Central Google Scholar * Tiffany ST, Conklin CA (2000). A cognitive processing model of alcohol
craving and compulsive alcohol use. _Addiction_ 95: 145–153. Article PubMed Google Scholar * Verdejo-Garcia A, Clark L, Dunn BD (2012). The role of interoception in addiction: a critical
review. _Neurosci Biobehav Rev_ 36: 1857–1869. Article PubMed Google Scholar * Vergara VM, Liu J, Claus ED, Hutchison K, Calhoun V (2017a). Alterations of resting state functional network
connectivity in the brain of nicotine and alcohol users. _Neuroimage_ 151: 45–54. Article CAS PubMed Google Scholar * Vergara VM, Mayer AR, Damaraju E, Hutchison K, Calhoun VD (2017b).
The effect of preprocessing pipelines in subject classification and detection of abnormal resting state functional network connectivity using group ICA. _Neuroimage_ 145 (Pt B): 365–376.
Article PubMed Google Scholar * Volkow ND, Baler RD, Compton WM, Weiss SR (2014). Adverse health effects of marijuana use. _N Engl J Med_ 370: 2219–2227. Article PubMed PubMed Central
Google Scholar * Warne RT (2014). A primer on multivariate analysis of variance (MANOVA) for behavioral scientists. _Pract Assess Res Eval_ 19. * Weiland BJ, Sabbineni A, Calhoun VD, Welsh
RC, Bryan AD, Jung RE _et al_ (2014). Reduced left executive control network functional connectivity is associated with alcohol use disorders. _Alcohol Clin Exp Res_ 38: 2445–2453. Article
PubMed PubMed Central Google Scholar * Williams LJ, Abdi H (2010) Fisher’s least significant difference (LSD) test Salkind NJ, Dougherty DM, Frey B (eds). _Encyclopedia of Research
Design_. SAGE Publications, Inc.: Thousand Oaks, CA, 218: 840–853. Google Scholar * Winstock AR (2014). The global drug survey 2014. findings. _Global Drug
Survey_http://www.globaldrugsurvey.com/facts-figures/the-global-drug-survey-2014-findings/. * Wise RA (2008). Dopamine and reward: the anhedonia hypothesis 30 years on. _Neurotox Res_ 14:
169–183. Article PubMed PubMed Central Google Scholar * Wise RA (2010). Neuroleptics and operant behavior: the anhedonia hypothesis. _Behav Brain Sci_ 5: 39. Article Google Scholar *
Wylie KP, Rojas DC, Tanabe J, Martin LF, Tregellas JR (2012). Nicotine increases brain functional network efficiency. _Neuroimage_ 63: 73–80. Article CAS PubMed Google Scholar * Yeh PH,
Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ (2009). Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational
neurocircuitry. _Psychiatry Res_ 173: 22–30. Article PubMed PubMed Central Google Scholar * Zalesky A, Solowij N, Yucel M, Lubman DI, Takagi M, Harding IH _et al_ (2012). Effect of
long-term cannabis use on axonal fibre connectivity. _Brain_ 135 (Pt 7): 2245–2255. Article PubMed Google Scholar * Zuckerman M (1996). Item revisions in the sensation seeking scale form
V (SSS-V). _Personality and Individual Differences_ 20: 515. Article Google Scholar Download references ACKNOWLEDGEMENTS We thank the staff of The Mind Research Network and of the
University of Colorado, Boulder, for their assistance with data integration and text editing, in particular Rupa Sabbineni, Brittny Miller, Ellen Blake, Christina Weywadt, and Eric Claus.
This work was supported by grants from the National Institutes of Health grant numbers 2R01EB005846, R01REB020407, and P20GM103472 to VDC, R01AA012238 and R01DA030344 to KEH, and National
Science Foundation (NSF) grants 1539067/1631819 to VDC. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * The Mind Research Network and Lovelace Biomedical and Environmental Research Institute,
Albuquerque, NM, USA Victor M Vergara & Vince D Calhoun * Departments of Psychology and Neuroscience, University of Colorado, Boulder, CO, USA Barbara J Weiland & Kent E Hutchison
Authors * Victor M Vergara View author publications You can also search for this author inPubMed Google Scholar * Barbara J Weiland View author publications You can also search for this
author inPubMed Google Scholar * Kent E Hutchison View author publications You can also search for this author inPubMed Google Scholar * Vince D Calhoun View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Victor M Vergara. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the
_Neuropsychopharmacology_ website SUPPLEMENTARY INFORMATION SUPPLEMENTARY MATERIAL (DOCX 248 KB) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE
FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Vergara, V., Weiland, B., Hutchison,
K. _et al._ The Impact of Combinations of Alcohol, Nicotine, and Cannabis on Dynamic Brain Connectivity. _Neuropsychopharmacol._ 43, 877–890 (2018). https://doi.org/10.1038/npp.2017.280
Download citation * Received: 15 May 2017 * Revised: 07 October 2017 * Accepted: 02 November 2017 * Published: 14 November 2017 * Issue Date: March 2018 * DOI:
https://doi.org/10.1038/npp.2017.280 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
